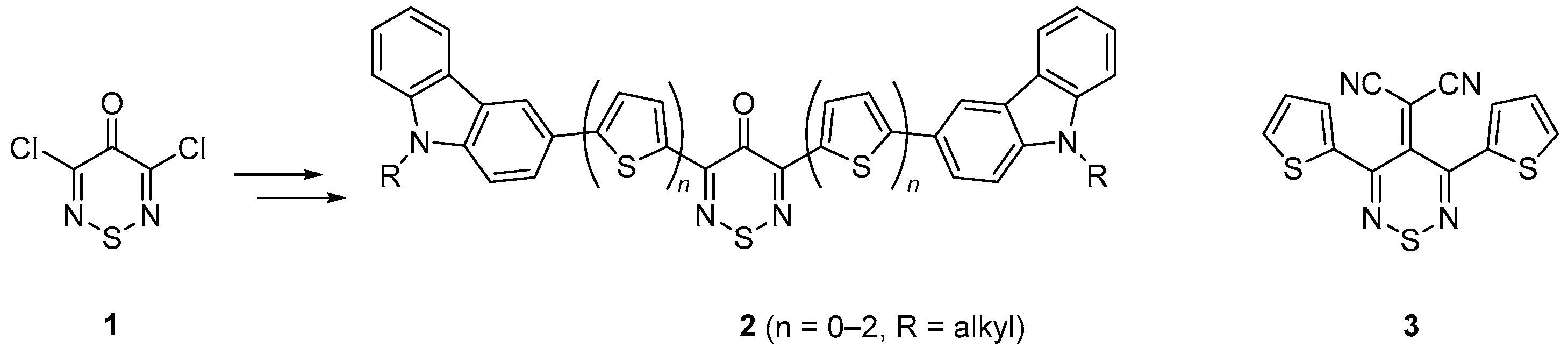
2-[3,5-Bis(5-bromothien-2-yl)-4H-1,2,6-thiadiazin-4-ylidene]-malononitrile
Abstract
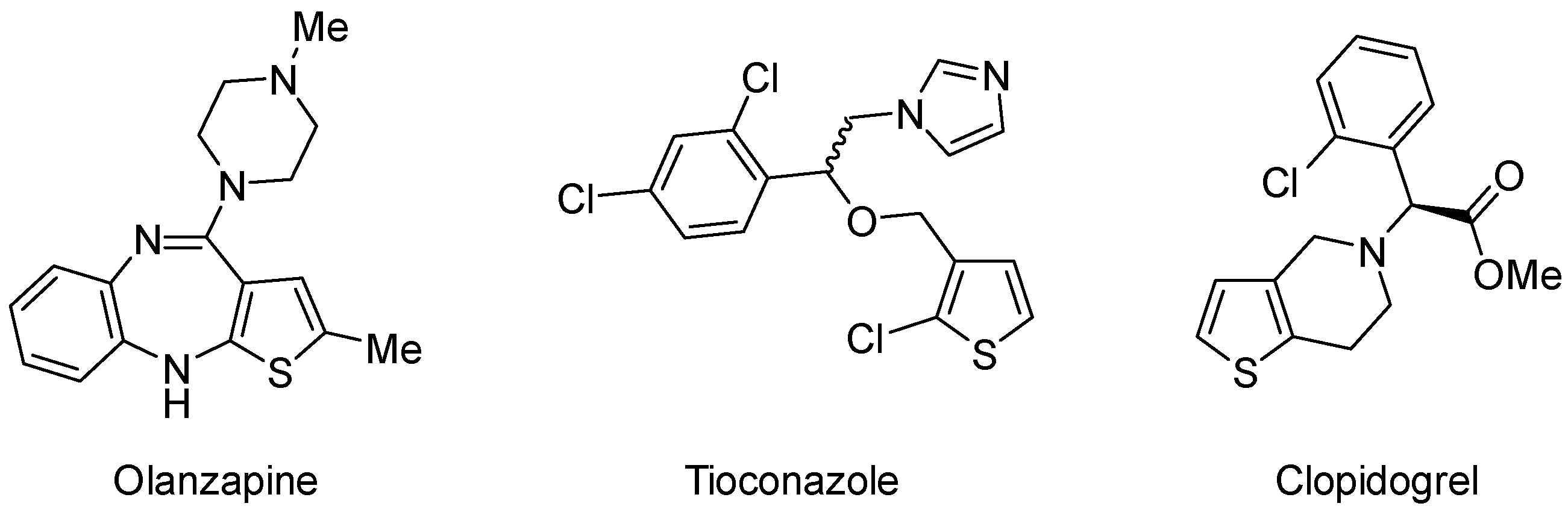
1. Introduction
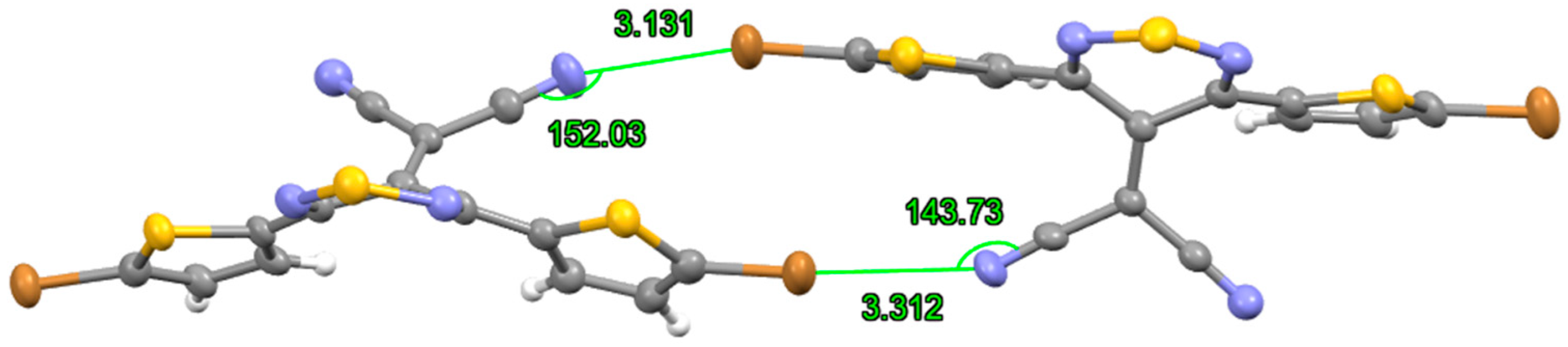
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gatti, T.; Lamberti, F.; Martí-Rujas, J.; Zheng, M. Thiophenes and Their Benzo Derivatives: Structure. In Comprehensive Heterocyclic Chemistry IV; StC Black, D., Cossy, J., Stevens, C.V., Eds.; Elsevier: Oxford, UK, 2022; Volume 3, Chapter 3.09; pp. 371–406. [Google Scholar] [CrossRef]
- Shao, X.; Chen, Y. Thiophenes and Their Benzo Derivatives: Applications. In Comprehensive Heterocyclic Chemistry IV; StC Black, D., Cossy, J., Stevens, C.V., Eds.; Elsevier: Oxford, UK, 2022; Volume 3, Chapter 3.12; pp. 613–652. [Google Scholar] [CrossRef]
- Caballero, R.; Cohen, B.; Gutiérrez, M. Thiophene-Based Covalent Organic Frameworks: Synthesis, Photophysics and Light-Driven Applications. Molecules 2021, 26, 7666. [Google Scholar] [CrossRef] [PubMed]
- Barbarella, G.; Melucci, M.; Sotgiu, G. The Versatile Thiophene: An Overview of Recent Research on Thiophene-Based Materials. Adv. Mater. 2005, 17, 1581–1593. [Google Scholar] [CrossRef]
- Groenendaal, L.B.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and Its Derivatives: Past, Present, and Future. Adv. Mater. 2000, 12, 481–494. [Google Scholar] [CrossRef]
- Hermerschmidt, F.; Kalogirou, A.S.; Min, J.; Zissimou, G.A.; Tuladhar, S.M.; Ameri, T.; Faber, H.; Itskos, G.; Choulis, S.A.; Anthopoulos, T.D.; et al. 4H-1,2,6-Thiadiazin-4-one-containing small molecule donors and additive effects on their performance in solution-processed organic solar cells. J. Mater. Chem. C 2015, 3, 2358–2365. [Google Scholar] [CrossRef]
- Chochos, C.L.; Kalogirou, A.S.; Ye, T.; Tatsi, E.; Katsouras, A.; Zissimou, G.A.; Gregoriou, V.G.; Avgeropoulos, A.; Koutentis, P.A. 4H-1,2,6-Thiadiazine-containing donor–acceptor conjugated polymers: Synthesis, optoelectronic characterization and their use in organic solar cells. J. Mater. Chem. C 2018, 6, 3658–3667. [Google Scholar] [CrossRef]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Economopoulos, S.P.; Koutentis, P.A.; Ioannidou, H.A.; Choulis, S.A. Identifying potential candidates for donor–acceptor copolymers on a series of 4H-1,2,6-thiadiazines: An electrochemical approach. Electrochim. Acta 2013, 107, 448–453. [Google Scholar] [CrossRef]
- Bird, C.W. A new aromaticity index and its application to five-membered ring heterocycles. Tetrahedron 1985, 41, 1409–1414. [Google Scholar] [CrossRef]
- Bird, C.W. The application of a new aromaticity index to six-membered ring heterocycles. Tetrahedron 1986, 42, 89–92. [Google Scholar] [CrossRef]
- Bird, C.W. Heteroaromaticity, 5, a unified aromaticity index. Tetrahedron 1992, 48, 335–340. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W.; White, A.J.P.; Williams, D.J. Reaction of tetracyanoethylene with SCl2: New molecular rearrangements. Chem. Commun. 2000, 1, 303–304. [Google Scholar] [CrossRef]
- Constantinides, C.P.; Carter, E.; Eisler, D.; Beldjoudi, Y.; Murphy, D.M.; Rawson, J.M. Effects of Halo-Substitution on 2′-Chloro-5′-halo-phenyl-1,2,3,5-dithiadiazolyl Radicals: A Crystallographic, Magnetic, and Electron Paramagnetic Resonance Case Study. Cryst. Growth Des. 2017, 17, 3017–3029. [Google Scholar] [CrossRef]
- Noland, W.E.; Schneerer, A.K.; Raberge, E.J.; Tritch, K.J. Crystal structure of 2,3,5,6-tetra bromo terephthalonitrile. Acta Cryst. E 2019, 75, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, C.P.; Berezin, A.A.; Estiva, G.; Early, F.B.; Zissimou, G.A.; Flesariu, F.D.; Lawson, D.B.; Manoli, M.; Leitus, G.; Koutentis, P.A. Ferromagnetic Interactions within a Dimer of a π-Extended 1,2,4-Benzotriazin-4-yl. Cryst. Growth Des. 2025, 25, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Bruker. Apex3, Saint; Bruker AXS Inc.: Madison, WI, USA, 2018. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Cryst. 2015, 71, 59–75. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Ioannidou, H.A.; Koutentis, P.A. Substitution at C-4 in 3,5-disubstituted 4H-1,2,6-thiadiazin-4-ones. Tetrahedron 2012, 68, 2590–2596. [Google Scholar] [CrossRef]



| Compound | EHOMODFT (eV) [a] | ELUMOTD-DFT (eV) [b] | EgTD-DFT (eV) [c] |
|---|---|---|---|
| 3 | −6.26 | −3.72 | 2.54 |
| 4 | −6.34 | −3.90 | 2.44 |
| 5 | −5.80 | −3.65 | 2.15 |
| 6 | −5.55 | −2.99 | 2.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalogirou, A.S.; Kourtellaris, A.; Koutentis, P.A. 2-[3,5-Bis(5-bromothien-2-yl)-4H-1,2,6-thiadiazin-4-ylidene]-malononitrile. Molbank 2025, 2025, M1977. https://doi.org/10.3390/M1977
Kalogirou AS, Kourtellaris A, Koutentis PA. 2-[3,5-Bis(5-bromothien-2-yl)-4H-1,2,6-thiadiazin-4-ylidene]-malononitrile. Molbank. 2025; 2025(1):M1977. https://doi.org/10.3390/M1977
Chicago/Turabian StyleKalogirou, Andreas S., Andreas Kourtellaris, and Panayiotis A. Koutentis. 2025. "2-[3,5-Bis(5-bromothien-2-yl)-4H-1,2,6-thiadiazin-4-ylidene]-malononitrile" Molbank 2025, no. 1: M1977. https://doi.org/10.3390/M1977
APA StyleKalogirou, A. S., Kourtellaris, A., & Koutentis, P. A. (2025). 2-[3,5-Bis(5-bromothien-2-yl)-4H-1,2,6-thiadiazin-4-ylidene]-malononitrile. Molbank, 2025(1), M1977. https://doi.org/10.3390/M1977







