(E)-3-Mesityl-1-(2,3,5,6-tetramethylphenyl)prop-2-en-1-one
Abstract
1. Introduction
2. Results and Discussion
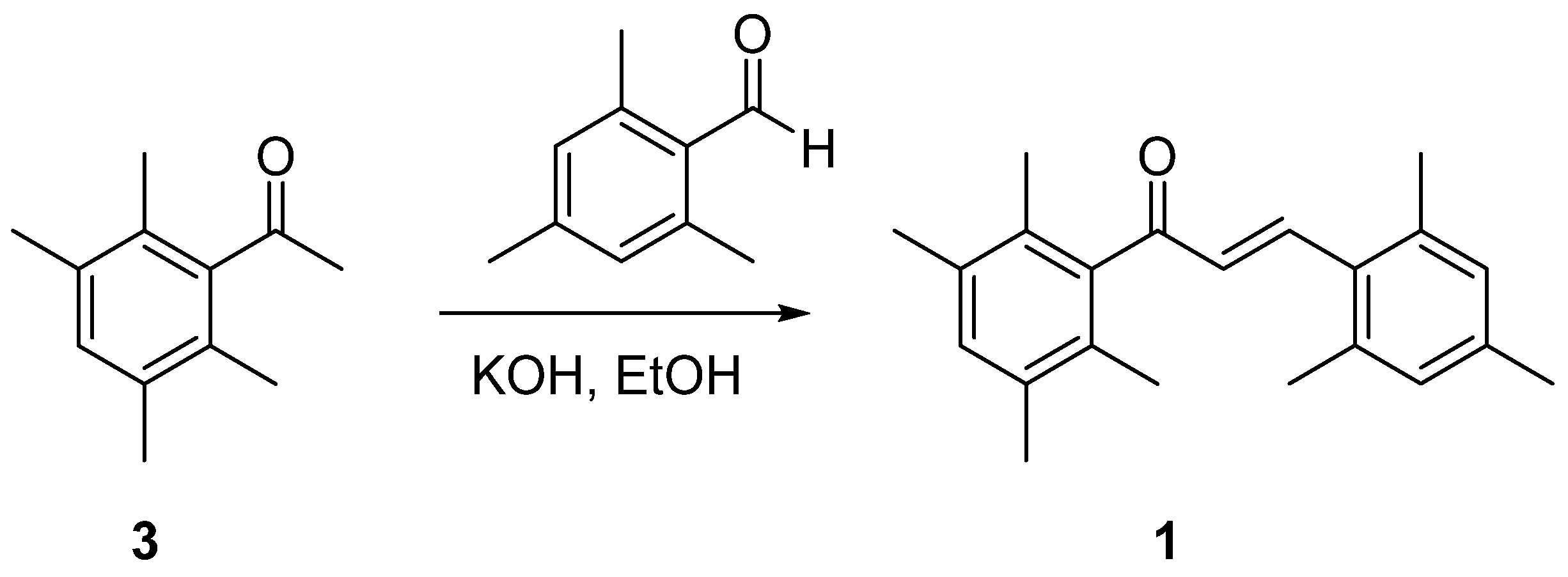
2.1. Synthesis and Spectroscopy
2.2. Structural Study
3. Materials and Methods
3.1. Synthesis of (E)-3-Mesityl-1-(2,3,5,6-tetramethylphenyl)prop-2-en-1-one (1)
3.2. X-Ray Crystallography
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuson, R.C.; Bannister, R.G. β-Duroylphenylpropionic Acids. J. Am. Chem. Soc. 1952, 74, 1629–1631. [Google Scholar] [CrossRef]
- Cheong, C.B.; Frost, J.R.; Donohoe, T.J. Pentamethylphenyl (Ph*) and Related Derivatives as Useful Acyl Protecting Groups for Organic Synthesis: A Preliminary Study. Synlett 2020, 31, 1828–1832. [Google Scholar] [CrossRef]
- Smith, L.I.; Guss, C. The Enolizing Action of Methylmagnesium Iodide upon Hindered Ketones. J. Am. Chem. Soc. 1937, 59, 804–806. [Google Scholar] [CrossRef]
- Fuson, R.C.; Meek, J.S. 1,4-Addition of the Grignard Reagent to Acetylenic Ketones. J. Org. Chem. 1945, 10, 551–561. [Google Scholar] [CrossRef]
- Armstrong, R.J.; Donohoe, T.J. Pentamethylphenyl (Ph*) ketones: Unique building blocks for organic synthesis. Tetrahedron Lett. 2021, 74, 153151–153159. [Google Scholar] [CrossRef]
- Sales, Z.S.; Nassar, R.; Morris, J.J.; Henderson, K.W. Stereoselective synthesis of enones from the reaction of aldehydes with sterically hindered dimethylaluminium enolates. J. Organomet. Chem. 2005, 690, 3474–3478. [Google Scholar] [CrossRef]
- Karplus, M. Vicinal Proton Coupling in Nuclear Magnetic Resonance. J. Am. Chem. Soc. 1963, 85, 2870–2871. [Google Scholar] [CrossRef]
- Rabinovich, D. Topochemistry. Part XXX. Crystal and molecular structures of chalcone. J. Chem. Soc. B 1970, 11–16. [Google Scholar] [CrossRef]
- Cordes, D.B.; Smellie, I.A.; Chalmers, B.A. (E)-1-(2,5-Dimethylphenyl)-3-phenylprop-2-en-1-one. Molbank 2024, 2024, M1862. [Google Scholar] [CrossRef]
- CrysAlisPro v1.171.42.109a; Rigaku Oxford Diffraction, Rigaku Corporation: Oxford, UK, 2024.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]




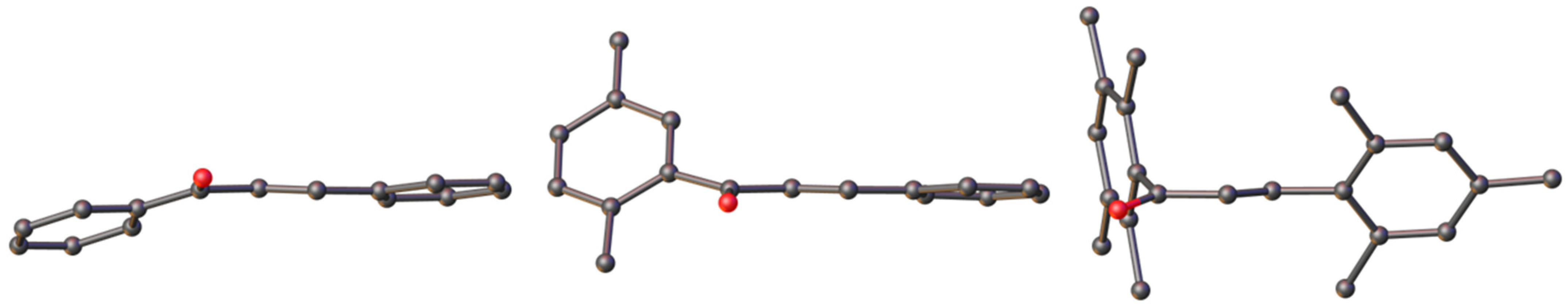



| BZYACO | ZOYWAP | 1 | |
|---|---|---|---|
| Bond Lengths | |||
| Alkene C=C | 1.319(6) | 1.326(3) | 1.3344(16) |
| C=O | 1.204(6) | 1.222(3) | 1.2243(14) |
| Torsion Angles | |||
| ring1−ring2 (a) | 11.4 | 56.2 | 75.28(4) |
| ring1−alkene | 21.0 | 50.4 | 84.34(4) |
| ring2−alkene | 9.8 | 6.3 | 54.91(14) |
| O1=C1−C2−H2 | 170.6 | 167.4 | 13.88(14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKay, A.P.; Cordes, D.B.; Smellie, I.A.; Chalmers, B.A. (E)-3-Mesityl-1-(2,3,5,6-tetramethylphenyl)prop-2-en-1-one. Molbank 2025, 2025, M1952. https://doi.org/10.3390/M1952
McKay AP, Cordes DB, Smellie IA, Chalmers BA. (E)-3-Mesityl-1-(2,3,5,6-tetramethylphenyl)prop-2-en-1-one. Molbank. 2025; 2025(1):M1952. https://doi.org/10.3390/M1952
Chicago/Turabian StyleMcKay, Aidan P., David B. Cordes, Iain A. Smellie, and Brian A. Chalmers. 2025. "(E)-3-Mesityl-1-(2,3,5,6-tetramethylphenyl)prop-2-en-1-one" Molbank 2025, no. 1: M1952. https://doi.org/10.3390/M1952
APA StyleMcKay, A. P., Cordes, D. B., Smellie, I. A., & Chalmers, B. A. (2025). (E)-3-Mesityl-1-(2,3,5,6-tetramethylphenyl)prop-2-en-1-one. Molbank, 2025(1), M1952. https://doi.org/10.3390/M1952








