(E)-N-(4-Methoxyphenyl)-1-(4′-(trifluoromethyl)-[1,1′-biphenyl]-4-yl)methanimine
Abstract
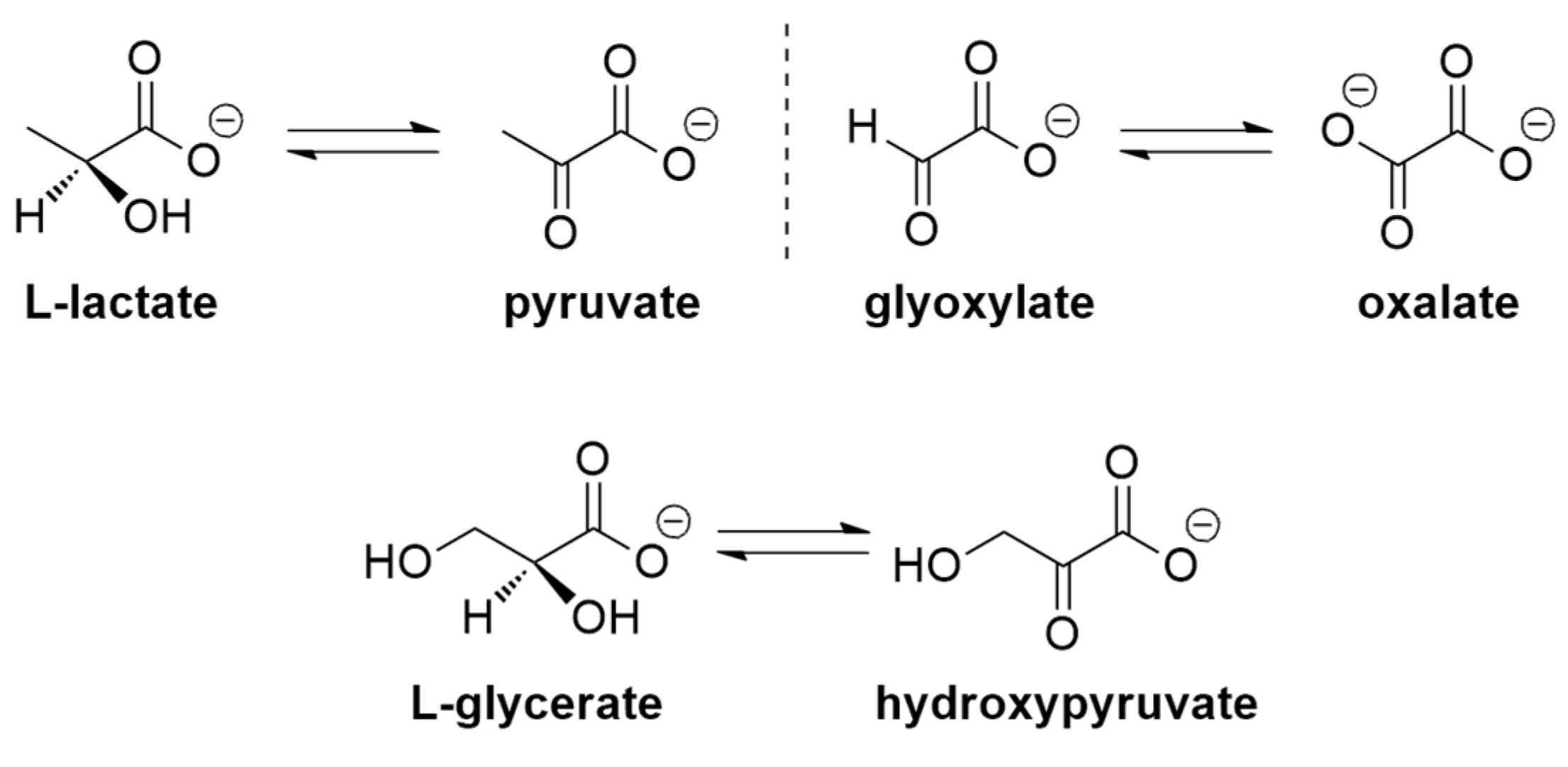
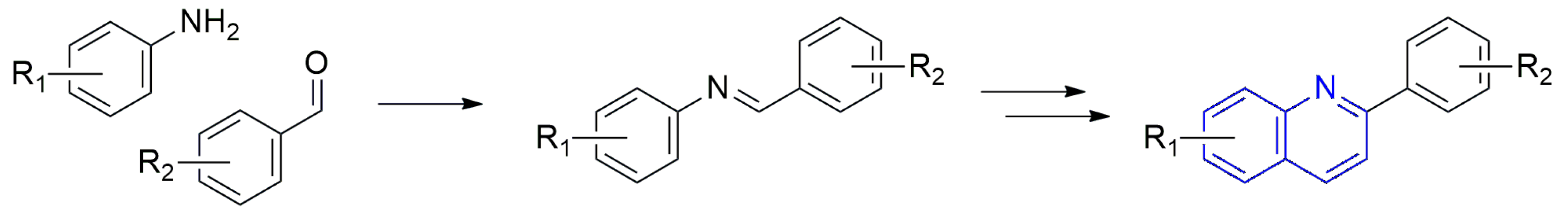
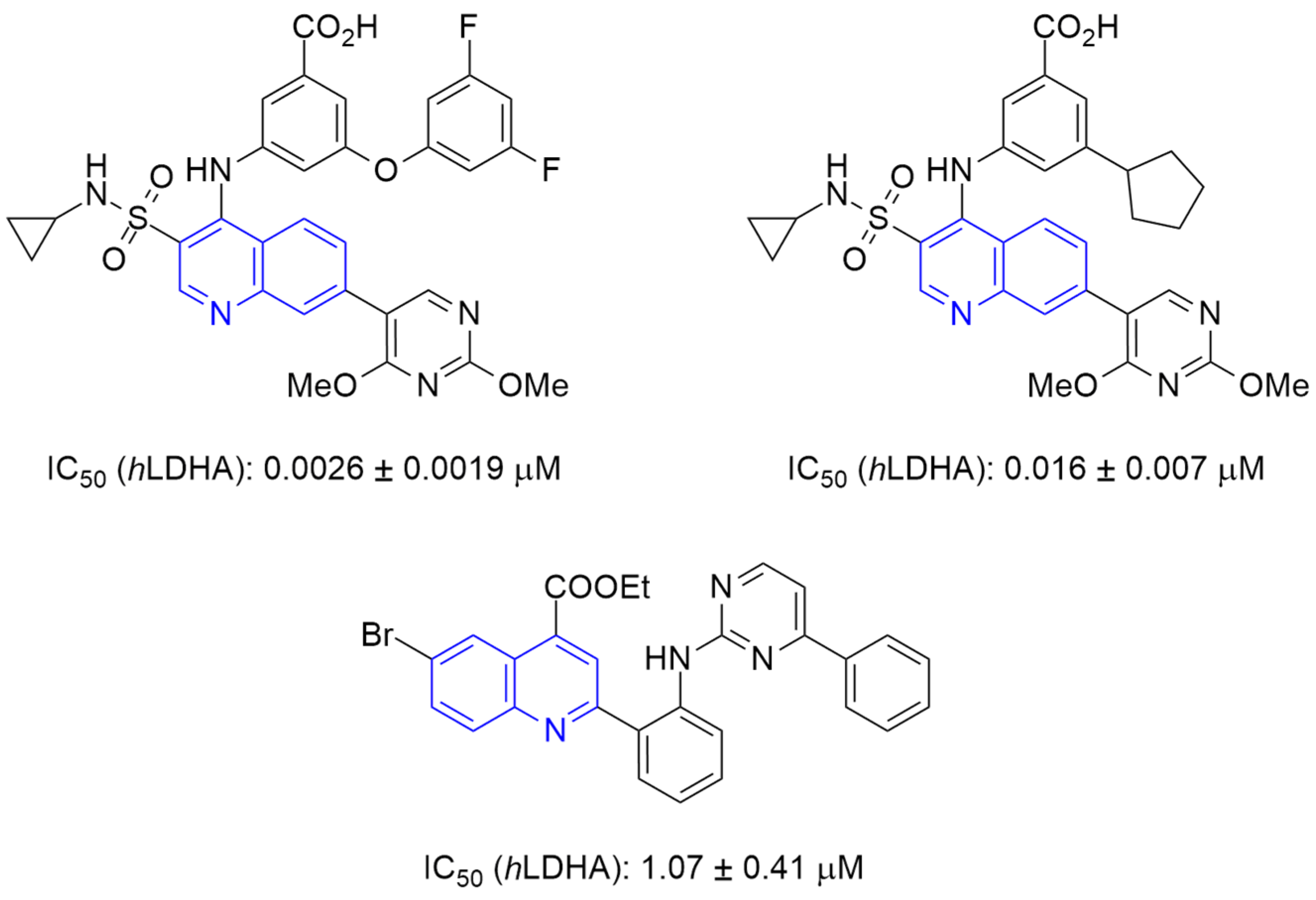
1. Introduction
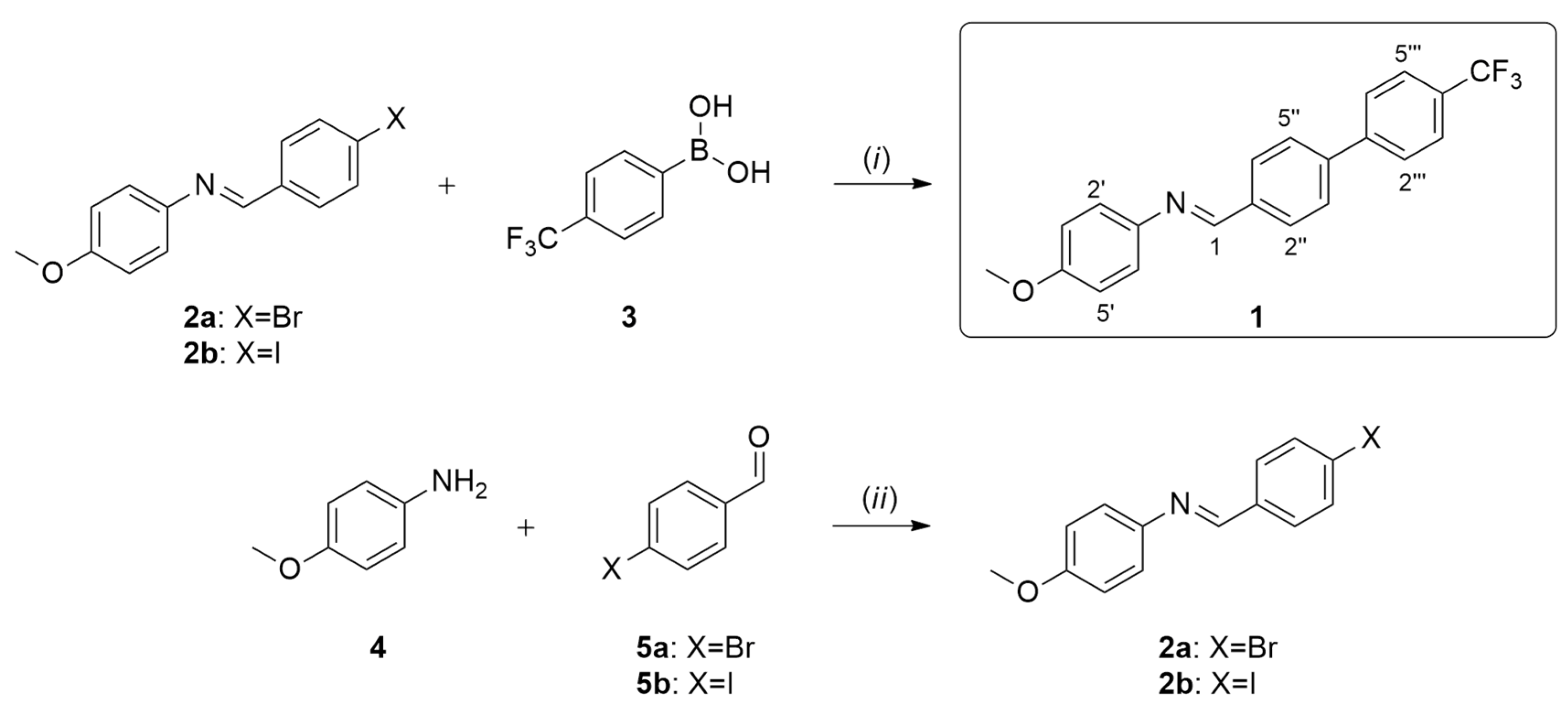
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Methods
3.2. Synthesis of Compound 1
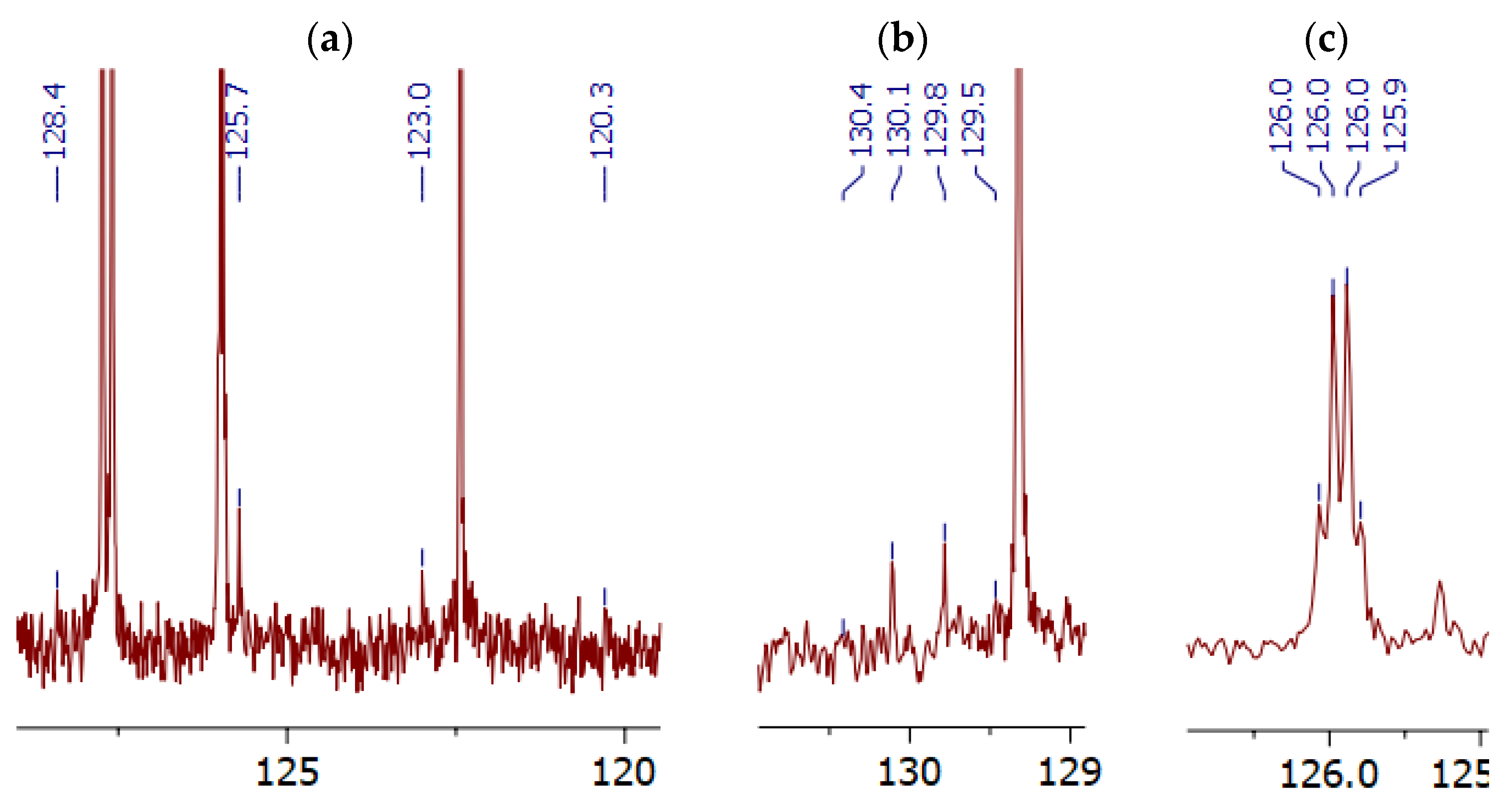
- •(E)-N-(4-methoxyphenyl)-1-(4′-(trifluoromethyl)-[1,1′-biphenyl]-4-yl)methanimine (1). Melting point: 190.4–191.2 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 8.54 (s, 1H, H-1), 7.98–8.01 (m, 2H, H-2″, H-6″), 7.69–7.76 (m, 6H, H-3″, H-5″, H-2‴, H-3‴, H-5‴, H-6‴), 7.26–7.30 (m, 2H, H-2′, H-6′), 6.94–6.98 (m, 2H, H-3′, H-5′), 3.85 (s, 3H, OCH3); 13C NMR (100 MHz, CDCl3) δ (ppm) 158.6 (C-4′), 157.6 (C-1), 144.8 (C-1′), 144.0 (C-4″), 142.2 (C-1‴), 136.4 (C-1″), 130.0 (q, J = 32.9 Hz, C-4‴), 129.3 (C-2″, C-6″), 127.7 (C-3″, C-5″)*, 127.6 (C-2‴, C-6‴)*, 126.0 (q, J = 3.7 Hz, C-3‴, C-5‴), 124.4 (q, J = 272.5 Hz, CF3), 122.4 (C-2′, C-6′), 114.6 (C-3′, C-5′), 55.7 (OCH3); * interchangeable signals. HRMS (EI) (M+) calc. for C21H16F3NO 355.11840, found 355.11804; m/z (%): 355.11804 (M+, 79), 340.09454 (M+–CH3, 100), 311.09204 (8), 285.08896 (M+–CF3, 12), 233.05765 (M+–C7H8NO, 5), 215.08588 (6), 167.55579 (4), 77.03868 (2). IR (cm−1): 3034 (aromatic C–H), 2922, 2853 (C–H), 1616 (C=N), 1574, 1504, 1464 (aromatic C=C), 1124 (C–F)*, 1111 (C–O)*, 837, 818 (aromatic C–H, p-substituted); * interchangeable signals. UV (EtOH), λmax (log ε): 289 (5.37), 350 (5.30) nm.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Han, J.H.; Lee, E.J.; Park, W.; Ha, K.T.; Chung, H.S. Natural compounds as lactate dehydrogenase inhibitors: Potential therapeutics for lactate dehydrogenase inhibitors-related diseases. Front. Pharmacol. 2023, 14, 1275000. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B.; Martín-Higueras, C. Improving treatment options for primary hyperoxaluria. Drugs 2022, 82, 1077–1094. [Google Scholar] [CrossRef] [PubMed]
- Granchi, C.; Paterni, I.; Rani, R.; Minutolo, F. Small-molecule inhibitors of human LDH5. Future Med. Chem. 2013, 5, 1967–1991. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Yang, F.; Li, Y. Natural products targeting human lactate dehydrogenases for cancer therapy: A mini review. Front. Chem. 2022, 10, 1013670. [Google Scholar] [CrossRef] [PubMed]
- Read, J.A.; Winter, V.J.; Eszes, C.M.; Sessions, R.B.; Brady, R.L. Structural basis for altered activity of M- and H-isozyme forms of human lactate dehydrogenase. Proteins 2001, 43, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Fiume, L.; Manerba, M.; Vettraino, M.; Di Stefano, G. Inhibition of lactate dehydrogenase activity as an approach to cancer therapy. Future Med. Chem. 2014, 6, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Claps, G.; Faouzi, S.; Quidville, V.; Chehade, F.; Shen, S.; Vagner, S.; Robert, C. The multiple roles of LDH in cancer. Nat. Rev. Clin. Oncol. 2022, 19, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Singh, M.; Rani, R. Role of LDH in tumor glycolysis: Regulation of LDHA by small molecules for cancer therapeutics. Semin. Cancer Biol. 2022, 87, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiong, Y.; Qiao, T.; Li, X.; Jia, L.; Han, Y. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018, 7, 6124–6136. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsov, V.V. Recent synthetic developments in a powerful imino Diels-Alder reaction (Povarov reaction): Application to the synthesis of N-polyheterocycles and related alkaloids. Tetrahedron 2009, 65, 2721–2750. [Google Scholar] [CrossRef]
- Özcan, E.; Ökten, S.; Eren, T. Decision making for promising quinoline-based anticancer agents through combined methodology. J. Biochem. Mol. Toxicol. 2020, 34, e22522. [Google Scholar] [CrossRef] [PubMed]
- Battista, S.; Marsicano, V.; Arcadi, A.; Galantini, L.; Aschi, M.; Allegrittini, E.; Del Giudice, A.; Giansanti, L. UV properties and loading into liposomes of quinoline derivatives. Colloids Interfaces 2021, 5, 28. [Google Scholar] [CrossRef]
- Billiard, J.; Dennison, J.B.; Briand, J.; Annan, R.S.; Chai, D.; Colón, M.; Dodson, C.S.; Gilbert, S.A.; Greshock, J.; Jing, J.; et al. Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab. 2013, 1, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Kumar, V. Recent update on human lactate dehydrogenase enzyme 5 (hLDH5) inhibitors: A promising approach for cancer chemotherapy. J. Med. Chem. 2016, 59, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Díaz, I.; Salido, S.; Nogueras, M.; Cobo, J. Synthesis of ethyl pyrimidine-quinolincarboxylates selected from virtual screening as enhanced lactate dehydrogenase (LDH) inhibitors. Int. J. Mol. Sci. 2024, 25, 9744. [Google Scholar] [CrossRef] [PubMed]
- Hurtová, M.; Biedermann, D.; Osifová, Z.; Cvačka, J.; Valentová, K.; Křen, V. Preparation of synthetic and natural derivatives of flavonoids using Suzuki-Miyaura cross-coupling reaction. Molecules 2022, 27, 967. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.T.; Yang, Y.J.; Lin, J.H.; Yang, D.Y. Fluorescence redox switches based on the opening and closing of an oxazabicyclic ring. Synlett 2010, 1, 111–114. [Google Scholar] [CrossRef][Green Version]
- Armarego, W.L.F. Purification of Laboratory Chemicals, 8th ed.; Butterworth-Heinemann: Burlington, UK, 2017. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rico-Molina, M.; Peña-García, D.; Altarejos, J.; Salido, S. (E)-N-(4-Methoxyphenyl)-1-(4′-(trifluoromethyl)-[1,1′-biphenyl]-4-yl)methanimine. Molbank 2025, 2025, M1953. https://doi.org/10.3390/M1953
Rico-Molina M, Peña-García D, Altarejos J, Salido S. (E)-N-(4-Methoxyphenyl)-1-(4′-(trifluoromethyl)-[1,1′-biphenyl]-4-yl)methanimine. Molbank. 2025; 2025(1):M1953. https://doi.org/10.3390/M1953
Chicago/Turabian StyleRico-Molina, Mario, David Peña-García, Joaquín Altarejos, and Sofía Salido. 2025. "(E)-N-(4-Methoxyphenyl)-1-(4′-(trifluoromethyl)-[1,1′-biphenyl]-4-yl)methanimine" Molbank 2025, no. 1: M1953. https://doi.org/10.3390/M1953
APA StyleRico-Molina, M., Peña-García, D., Altarejos, J., & Salido, S. (2025). (E)-N-(4-Methoxyphenyl)-1-(4′-(trifluoromethyl)-[1,1′-biphenyl]-4-yl)methanimine. Molbank, 2025(1), M1953. https://doi.org/10.3390/M1953






