Crystal Structure of 5-Nitro-4-(4-methoxyphenoxy)phthalonitrile
Abstract
1. Introduction
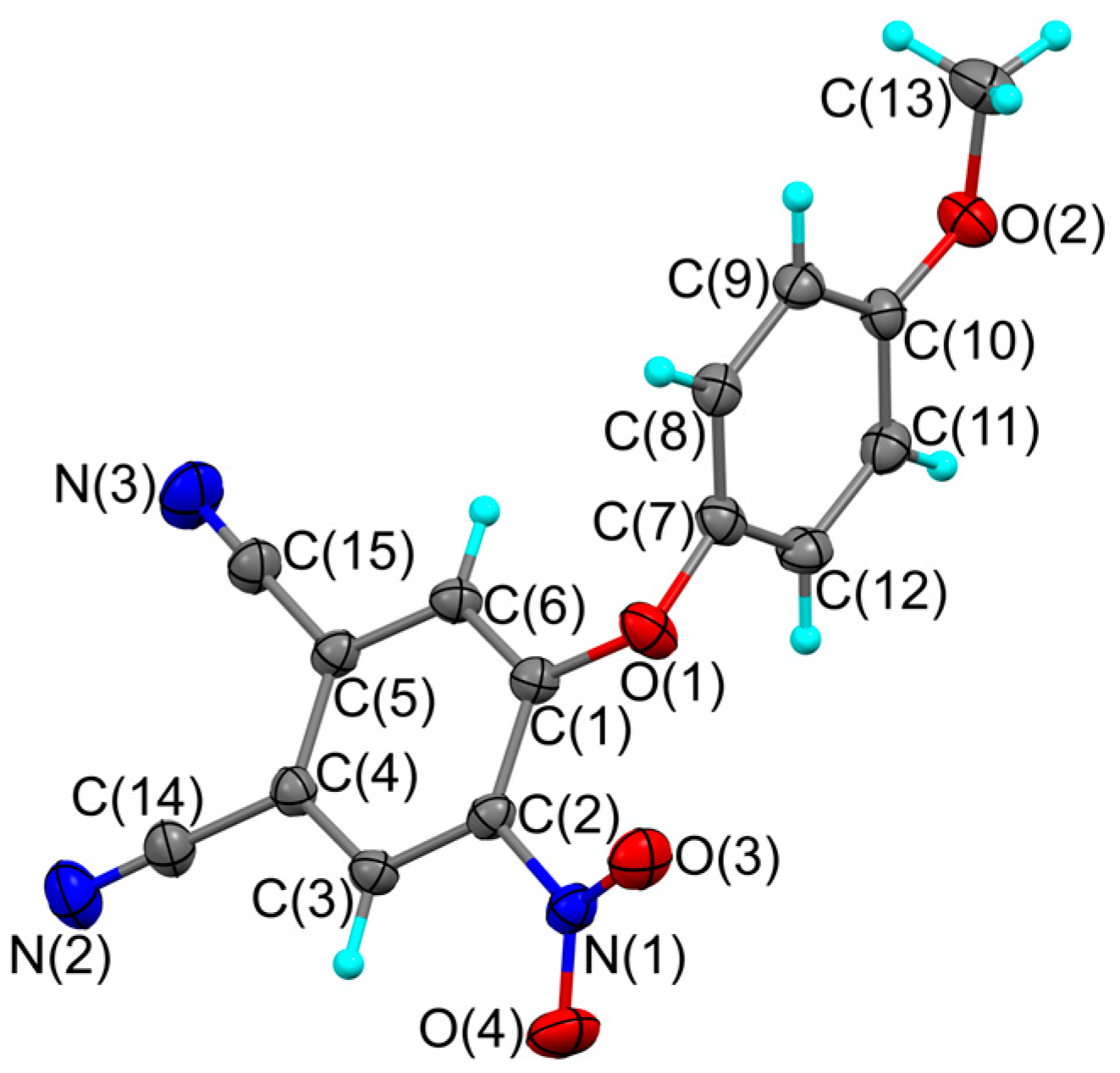
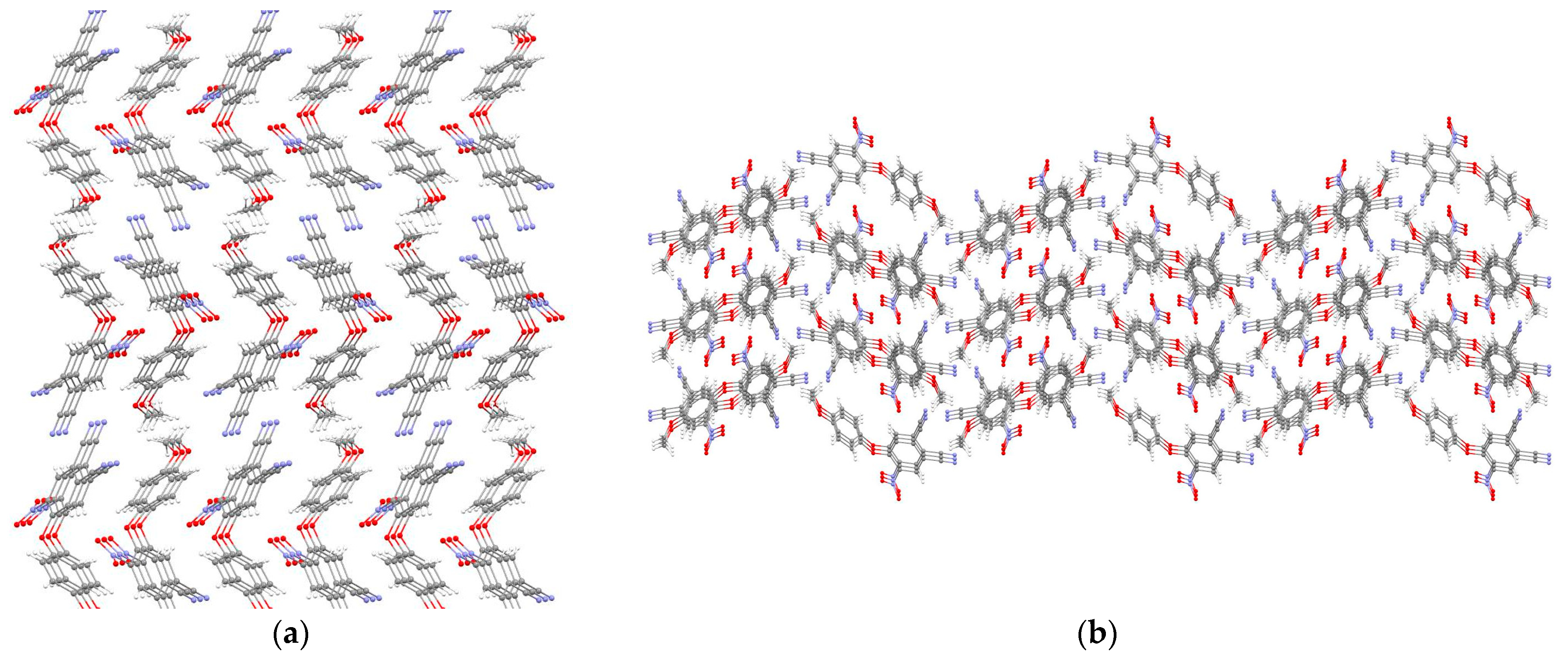
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of 5-Nitro-4-(4-methoxyphenoxy)phthalonitrile
3.2. X-Ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vashurin, A.; Maizlish, V.; Tikhomirova, T.; Nemtseva, M.; Znoyko, S.; Aleksandriiskii, V. Novel Non-Symmetrical Bifunctionally-Substituted Phthalonitriles and Corresponding d-Metal Phthalocyaninates. J. Mol. Struct. 2018, 1160, 440–446. [Google Scholar] [CrossRef]
- Derradji, M.; Wang, J.; Liu, W. bin High Performance Ceramic-Based Phthalonitrile Micro and Nanocomposites. Mater. Lett. 2016, 182, 380–385. [Google Scholar] [CrossRef]
- Bulgakov, B.A.; Morozov, O.S.; Timoshkin, I.A.; Babkin, A.V.; Kepman, A.V. Bisphthalonitrile-Based Thermosets as Heat-Resistant Matrices for Fiber Reinforced Plastics. Polym. Sci. Ser. C 2021, 63, 64–101. [Google Scholar] [CrossRef]
- Bulgakov, B.A.; Schubert, U.S. Recent Advances and Prospects in High-Performance Bio-Based Phthalonitrile Resins. Adv. Funct. Mater. 2024, 34, 2410071. [Google Scholar] [CrossRef]
- Keller, T.M. Imide-Containing Phthalonitrile Resin. Polymer 1993, 34, 952–955. [Google Scholar] [CrossRef]
- Laskoski, M.; Dominguez, D.D.; Keller, T.M. Synthesis and Properties of a Bisphenol A Based Phthalonitrile Resin. J. Polym. Sci. A Polym. Chem. 2005, 43, 4136–4143. [Google Scholar] [CrossRef]
- Phya, E.J.R.; Liu, M.; Lim, J.S.K.; Cho, B.; Gan, C.L. Phthalonitrile -Based Electonic Pakages for High Temperature Applications. In Proceedings of the 2018 IEEE 20th Electronics Packaging Technology Conference (EPTC), Singapore, 4–7 December 2018; p. 537. [Google Scholar]
- Kobayashi, N. Phthalocyanines. Curr. Opin. Solid State Mater. Sci. 1999, 4, 345–353. [Google Scholar] [CrossRef]
- Kobayashi, N.; Fukuda, T. Recent Progress in Phthalocyanine Chemistry: Synthesis and Characterization. In Functional Dyes; Kim, S.-H., Ed.; Elsiever: Amsterdam, The Netherlands, 2006; pp. 1–45. ISBN 9780444521767. [Google Scholar] [CrossRef]
- Erzunov, D.; Rassolova, A.; Botnar, A.; Tonkova, S.; Rumyantsev, R.; Maizlish, V.; Aleksandriskii, V.; Vashurin, A. The Influence of Methoxy- Group Position on Thermal Stability and Properties of Novel Isomeric 4-[(Methoxy)Phenoxy] Phthalonitriles and Phthalocyanine Complexes Based on Them. Dyes Pigments 2023, 219, 111600. [Google Scholar] [CrossRef]
- Erzunov, D.; Rassolova, A.; Rumyantsev, R.; Maizlish, V.; Tikhomirova, T.; Vashurin, A. Crystal Structures of 4-(2/3-Methoxyphenoxy)-Phthalonitrile. Acta Crystallogr. E Crystallogr. Commun. 2023, 79, 172–176. [Google Scholar] [CrossRef]
- Vatsanov, S.S. Van Der Waals Radii of Elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Baykov, S.V.; Filimonov, S.I.; Rozhkov, A.V.; Novikov, A.S.; Ananyev, I.V.; Ivanov, D.M.; Kukushkin, V.Y. Reverse Sandwich Structures from Interplay between Lone Pair-π-Hole Atom-Directed C···dz2[M] and Halogen Bond Interactions. Cryst. Growth Des. 2020, 20, 995–1008. [Google Scholar] [CrossRef]
- Jan, C.Y.; Shamsudin, N.B.H.; Tan, A.L.; Young, D.J.; Ng, S.W.; Tiekink, E.R.T. 4-Nitrophthalonitrile. Acta Crystallogr. Sect. E Struct. Rep. Online 2014, 70, o323. [Google Scholar] [CrossRef] [PubMed]
- Köç, M.; Zorlu, Y.; Işci, Ü.; Berber, S.; Ahsen, V.; Dumoulin, F. A Library of Dimeric and Trimeric Phthalonitriles Linked by a Single Aromatic Ring: Comparative Structural and DFT Investigations. CrystEngComm 2016, 18, 1416–1426. [Google Scholar] [CrossRef]
- Janiak, C. A Critical Account on N-n Stacking in Metal Complexes with Aromatic Nitrogen-Containing Ligands. J. Chem. Soc. Dalton Trans. 2000, 21, 3885–3896. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System. Rigaku J. 2016, 32, 31–34. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]


| D–H···A/D···A | D–H | H···A | D···A | D–H···A |
|---|---|---|---|---|
| O1···O3 | N/A | N/A | 2.618(2) | N/A |
| C3-H3A···N2 i,* | 0.93 | 2.69 | 3.600(3) | 166.8 |
| C9-H9A···N3 ii | 0.93 | 2.64 | 3.550(2) | 165.2 |
| C12-H12A···O4 iii | 0.93 | 2.51 | 3.276(2) | 140.0 |
| C2···C11 iv | N/A | N/A | 3.627(2) | N/A |
| C5···C8 v | N/A | N/A | 3.352(2) | N/A |
| O2···O3 v | N/A | N/A | 3.999(2) | N/A |
| O2···O4 v | N/A | N/A | 3.752(3) | N/A |
| O1···O4 vi | N/A | N/A | 3.773(2) | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erzunov, D.; Rassolova, A.; Abramov, I.; Maizlish, V.; Rumyantsev, R.; Vashurin, A. Crystal Structure of 5-Nitro-4-(4-methoxyphenoxy)phthalonitrile. Molbank 2025, 2025, M1946. https://doi.org/10.3390/M1946
Erzunov D, Rassolova A, Abramov I, Maizlish V, Rumyantsev R, Vashurin A. Crystal Structure of 5-Nitro-4-(4-methoxyphenoxy)phthalonitrile. Molbank. 2025; 2025(1):M1946. https://doi.org/10.3390/M1946
Chicago/Turabian StyleErzunov, Dmitry, Anastasia Rassolova, Igor Abramov, Vladimir Maizlish, Roman Rumyantsev, and Arthur Vashurin. 2025. "Crystal Structure of 5-Nitro-4-(4-methoxyphenoxy)phthalonitrile" Molbank 2025, no. 1: M1946. https://doi.org/10.3390/M1946
APA StyleErzunov, D., Rassolova, A., Abramov, I., Maizlish, V., Rumyantsev, R., & Vashurin, A. (2025). Crystal Structure of 5-Nitro-4-(4-methoxyphenoxy)phthalonitrile. Molbank, 2025(1), M1946. https://doi.org/10.3390/M1946







