Octahedral Oxo-Bridged Tri-Nickel(II) Complex with 1,3,5-Tris(2-hydroxyphenyl)-2,4-diazapenta-1,3-diene
Abstract
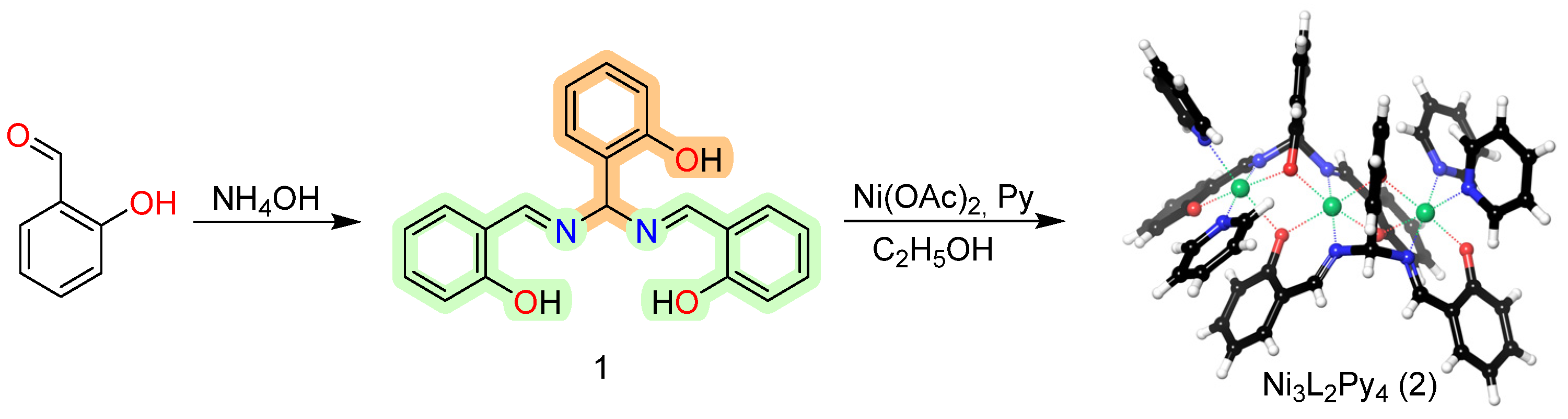
1. Introduction
2. Results
3. Materials and Methods
3.1. Synthesis of 1,3,5-Tris(2-hydroxyphenyl)-2,4-diazapentadiene (1)
3.2. Synthesis of Ni3L2Py4 (2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Poddar, S.N. Complex Compounds of Schiff’s Bases of 3-Aldehydo-Salicylic Acid. Z. Anorg. Allg. Chem. 1963, 322, 326–336. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, N.; Li, C.Q.; Shi, W.G.; Lin, Z.Y. Nickel Complexes Based on Hyperbranched Salicylaldimine Ligands: Synthesis, Characterization, and Catalytic Properties for Ethylene Oligomerization. J. Organomet. Chem. 2016, 822, 104–111. [Google Scholar] [CrossRef]
- Chen, L.; Huo, H.; Wang, L.; Ma, L.; Jiang, Y.; Xie, J.; Wang, J. Nickel Complexes Based on Salicylaldehyde-Imine Ligands: Synthesis, Characterization and Catalytic Oligomerization of Ethylene. Chem. Res. Chin. Univ. 2018, 34, 945–951. [Google Scholar] [CrossRef]
- Vereschagin, A.A.; Sizov, V.V.; Vlasov, P.S.; Alekseeva, E.V.; Konev, A.S.; Levin, O.V. Water-Stable [Ni(Salen)]-Type Electrode Material Based on Phenylazosubstituted Salicylic Aldehyde Imine Ligand. New J. Chem. 2017, 41, 13918–13928. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.D.; Chavooshi, D.; Descher, H.A.; Leitner, D.; Talasz, H.; Hermann, M.; Wurst, K.; Hohloch, S.; Gust, R.; Kircher, B. Design, Synthesis, Electrochemical, and Biological Evaluation of Fluorescent Chlorido[N,N′-Bis(Methoxy/Hydroxy)Salicylidene-1,2-Bis(4-Methoxyphenyl)Ethylenediamine]Iron(III) Complexes as Anticancer Agents. J. Med. Chem. 2023, 66, 15916–15925. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.H.; Pandya, N.; Kureshy, R.I.; Abdi, S.H.R.; Agrawal, S.; Bajaj, H.C.; Pandya, J.; Gupte, A. Synthesis, Characterization, DNA Binding and Cleavage Studies of Chiral Ru(II) Salen Complexes. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2009, 74, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Sprung, M.A. A Summary of the Reactions of Aldehydes with Amines. Chem. Rev. 1940, 26, 297–338. [Google Scholar] [CrossRef]
- Rigamonti, L.; Zardi, P.; Carlino, S.; Demartin, F.; Castellano, C.; Pigani, L.; Ponti, A.; Ferretti, A.M.; Pasini, A. Selective Formation, Reactivity, Redox and Magnetic Properties of MnIII and FeIII Dinuclear Complexes with Shortened Salen-Type Schiff Base Ligands. Int. J. Mol. Sci. 2020, 21, 7882. [Google Scholar] [CrossRef] [PubMed]
- Chiari, B.; Cinti, A.; Crispu, O.; Demartin, F.; Pasini, A.; Piovesana, O. Binuclear Co(Ii)Co(Ii), Co(Ii)Co(Iii) and Co(Iii)Co(Iii) Complexes of “Short” Salen Homologues Derived from the Condensation of Salicylaldehyde and Methanediamine or Phenylmethanediamines. Synthesis, Structures and Magnetism. J. Chem. Soc. Dalton Trans. 2001, 3611–3616. [Google Scholar] [CrossRef]
- Batley, G.; Graddon, D. Nickel(II) Hydrosalicylamide Complexes. Aust. J. Chem. 1967, 20, 1749–1751. [Google Scholar] [CrossRef]
- Le Phuong, H.A.; Cseri, L.; Whitehead, G.F.S.; Garforth, A.; Budd, P.; Szekely, G. Environmentally Benign and Diastereoselective Synthesis of 2,4,5-Trisubstituted-2-Imidazolines. RSC Adv. 2017, 7, 53278–53289. [Google Scholar] [CrossRef]
- Lozinskaya, N.A.; Tsybezova, V.V.; Proskurnina, M.V.; Zefirov, N.S. Regioselective Synthesis of Cis- and Trans-2,4,5-Triarylimidazolines and 2,4,5-Triarylimidazoles from Available Reagents. Russ. Chem. Bull. 2003, 52, 674–678. [Google Scholar] [CrossRef]
- Kamal, A.; Ahmad, A.; Qureshi, A.A. Syntheses of Some Substituted Hexamines in Aqueous Medium. Tetrahedron 1963, 19, 869–872. [Google Scholar] [CrossRef]
- Bazanov, D.R.; Avdeev, Y.G.; Lozinskaya, N.A.; Andreeva, T.E.; Kuznetsov, Y.I. Alkylphenyl-Substituted Imidazolines as Corrosion Inhibitors: Experimental and DFT Study. Mendeleev Commun. 2024, 34, 751–754. [Google Scholar] [CrossRef]
- Bazanov, D.R.; Pervushin, N.V.; Savitskaya, V.Y.; Anikina, L.V.; Proskurnina, M.V.; Lozinskaya, N.A.; Kopeina, G.S. 2,4,5-Tris(Alkoxyaryl)Imidazoline Derivatives as Potent Scaffold for Novel P53-MDM2 Interaction Inhibitors: Design, Synthesis, and Biological Evaluation. Bioorg. Med. Chem. Lett. 2019, 29, 2364–2368. [Google Scholar] [CrossRef] [PubMed]
- Bazanov, D.R.; Lozinskaya, N.A. Diastereoselective Synthesis of Meso-1,2-Diarylethane-1,2-Diamines via Sodium Reduction of Imidazolines. Asian J. Org. Chem. 2024, 13, e202400305. [Google Scholar] [CrossRef]
- Bernès, S. Redetermination of Bis(2-Formylphenolato-κ2O,O′)Nickel(II) as Bis[2-(Iminomethyl)Phenolato-κ2N,O′]Nickel(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, m100. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, A.A.; Eremin, A.V.; Gurzhii, V.V.; Misharev, A.D.; Medvedskii, N.L.; Ponyaev, A.I.; Belyaev, A.N. Syntheses and Structural Studies of the Nickel(II) Octahedral Complexes Ni(N∩N)xL2 with Nitrogen-Containing and Carboxylate Ligands. Russ. J. Coord. Chem. 2017, 43, 269–277. [Google Scholar] [CrossRef]
- Jana, K.; Maity, T.; Chandra Debnath, S.; Samanta, B.C.; Seth, S.K. Octahedral Ni(II) Complex with New NNO Donor Schiff Base Ligand: Synthesis, Structure and Hirshfeld Surface. J. Mol. Struct. 2017, 1130, 844–854. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazanov, D.R.; Korolyov, E.D.; Lyssenko, K.A.; Lozinskaya, N.A. Octahedral Oxo-Bridged Tri-Nickel(II) Complex with 1,3,5-Tris(2-hydroxyphenyl)-2,4-diazapenta-1,3-diene. Molbank 2025, 2025, M1945. https://doi.org/10.3390/M1945
Bazanov DR, Korolyov ED, Lyssenko KA, Lozinskaya NA. Octahedral Oxo-Bridged Tri-Nickel(II) Complex with 1,3,5-Tris(2-hydroxyphenyl)-2,4-diazapenta-1,3-diene. Molbank. 2025; 2025(1):M1945. https://doi.org/10.3390/M1945
Chicago/Turabian StyleBazanov, Daniil R., Egor D. Korolyov, Konstantin A. Lyssenko, and Natalia A. Lozinskaya. 2025. "Octahedral Oxo-Bridged Tri-Nickel(II) Complex with 1,3,5-Tris(2-hydroxyphenyl)-2,4-diazapenta-1,3-diene" Molbank 2025, no. 1: M1945. https://doi.org/10.3390/M1945
APA StyleBazanov, D. R., Korolyov, E. D., Lyssenko, K. A., & Lozinskaya, N. A. (2025). Octahedral Oxo-Bridged Tri-Nickel(II) Complex with 1,3,5-Tris(2-hydroxyphenyl)-2,4-diazapenta-1,3-diene. Molbank, 2025(1), M1945. https://doi.org/10.3390/M1945






