Abstract
The oxidative esterification of 4,4′-Biphenyldicarboxaldehyde with hexafluoroisopropanol is reported. The methodology, making a novel hexafluoroisopropyl ester uses a green, recyclable oxoammonium salt along with easily obtained sodium persulfate.
1. Introduction
Oxidation reactions are of utmost importance to the field of chemistry. The most common oxidizing agents, however, contain chromium and manganese. Though these are essential nutrients, larger quantities can be harmful to the environment, especially to marine organisms [1]. Due to the toxicity of typical oxidants, the need for greener alternatives is apparent. One such alternative is a group of oxoammonium oxidants, the most popular of which is 4-acetamido-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate (AcNH-TEMPO+BF4−, 1) (Figure 1). This compound is metal-free and recyclable, which are ever-important factors in the field of green chemistry (For a review, see [2]). However, although 1 is greener than conventional oxidants, when operating in basic medium it is typically employed in a superstoichiometric quantity. This is because the depleted oxidant, hydroxylammonium salt 2, undergoes a comproportionation reaction with active oxidant 1 to generate nitroxide 3, thereby requiring sacrificial amounts of 1 to perform oxidation reactions. Our group discovered that sodium persulfate can be used to generate the active oxoammonium form 1 from nitroxide 3 using sulfate radicals, which allows the usage of the latter in catalytic amounts (For an example, see: [3]).
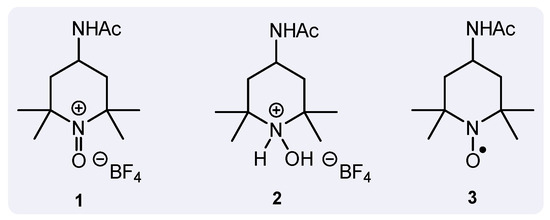
Figure 1.
Oxoammonium salt (1), along with the hydroxylammonium (2) and nitroxide (3) analogs.
Our group has worked quite extensively on oxidation reactions employing oxoammonium salts, starting with the selective oxidation of alcohols (For a recent example, see: [4]). However, perhaps more interesting from a chemical-space perspective are oxidative functionalization reactions, which provide the opportunity to generate classes of compounds such as amides, nitriles, and esters (Figure 2). In the case of the latter, hexafluoroisopropyl (HFIP) esters are particularly valuable compounds both in their own right and also as starting materials for further functional group transformations. HFIP esters are more lipophilic than traditional esters due to the trifluoromethyl groups [5]. They readily react to form various different carboxylic acid derivatives, such as amides or other esters. The synthesis of HFIP esters can be somewhat challenging as they are easily hydrolyzed. Our initial methodology involved the use of 1 in a superstoichiometric quantity (Figure 2a) [6]. However, more recently, we have developed catalytic approaches using 3, one being visible light-driven (Figure 2b) and another employing sodium persulfate as a co-oxidant (Figure 2c). Using the latter, we have prepared a wide range of HFIP esters, all having one HFIP moiety attached. In this report, we present the synthesis, isolation, and characterization of a bis-HFIP ester, 5, from dialdehyde 4, thereby further showcasing the methodology (Figure 3).
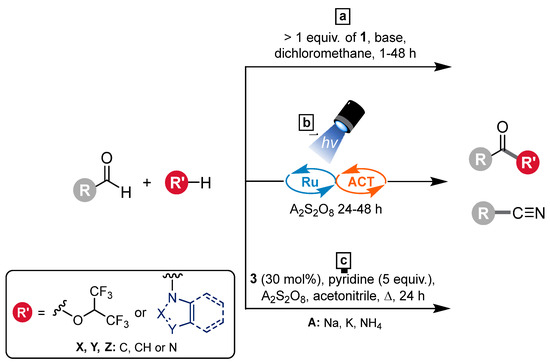
Figure 2.
Oxidative functionalization reactions: (a) using a superstoichiometric quantity of oxoammonium salt; (b) merging oxoammonium cation and visible light photocatalysis; (c) using a persulfate salt.
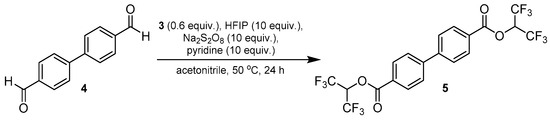
Figure 3.
Preparation of bis(1,1,1,3,3,3-hexafluoropropan-2-yl) [1,1′-biphenyl]-4,4′-dicarboxylate (5) from 4,4′-biphenyldicarboxaldehyde (4).
2. Results and Discussion
To prepare the bis-HFIP ester 5, we first used our optimized reaction conditions from our previously published approach to mono-HFIP functionalized esters [6]. The relative stoichiometry of pyridine, sodium persulfate, HFIP, and 3 were doubled to account for the formation of two ester groups. We were pleased to find that further optimization was not required. Using 1 equiv. of 4,4′-biphenyldicarboxaldehyde 4, 10 equiv. each of pyridine, sodium persulfate and HFIP and heating at 50 °C for 24 h led to a 76% conversion to the desired bis-HFIP ester 5. The reaction time was extended to 48 h in an attempt to increase conversion, but no improvement was observed. Isolation then became the next goal.
The product isolation stage was initiated by using the procedure that had proven success for the monofunctionalized HFIP esters we prepared previously with two modifications: the removal of a base wash step and usage of hexane rather than pentane for the aqueous/organic extraction. Pentane, which is less desirable than hexane, was utilized in the original method due to the high volatility of a number of the HFIP ester products. Since 5 is a solid at room temperature, hexane was used instead. Thus, our process involved adding hexane to the reaction vessel and decanting the solution into a separatory funnel. After repeating this process a further two times, we washed the combined hexane component with water and then removed the aqueous layer before adding 0.5 M hydrochloric acid. After shaking, the aqueous layer was removed and the organic layer was dried to afford the product, 5 in 46% yield.
We characterized the new bis-HFIP ester product (5) using a range of spectroscopic techniques. The 1H-NMR shows a characteristic septet for the proton associated with the HFIP moiety, along with two doublets associated with the aromatic rings. The 19F-NMR shows a doublet, corresponding to the four trifluoromethyl groups of the HFIP group. The 13C-NMR shows seven carbon environments; of note is the septet associated with the carbon attached to the CF3 groups, as well as a quartet correlating to the CF3 carbons. The IR spectrum has a sharp signal at 1740 cm−1 which is attributed to the stretch of the ester carbonyl bond. Notably, there is not an O-H stretch present, thus confirming that there is not any contamination from hydrolysis of the ester. Unfortunately, the product is not amenable to electrospray ionization (ESI) high-resolution mass spectrometry, the predominant signal observed being from the hydrolysis product. This is not unprecedented, as this artifact has been reported before [7]. However, we were successful when using accurate mass electron impact ionization [8].
3. Materials and Methods
3.1. General Experimental
1H and 19F NMR spectra were performed at 300 K on a Bruker 500 MHz spectrometer. 13C NMR spectrum was performed at 300 K on a Bruker 300 MHz spectrometer. 1H-NMR spectra were referenced to residual CHCl3 (7.26 ppm) in CDCl3. 13C-NMR spectra were referenced to CDCl3 (77.16 ppm). 19F-NMR spectra were referenced to hexafluorobenzene (−164.9 ppm). Reactions were monitored by 1H-NMR, and/or by TLC on silica gel plates (60 Å porosity, 250 μm thickness). TLC analysis was performed using UV light. Accurate mass electron impact ionization (EI) data were obtained using an Agilent 7820A-5975 gas chromatograph–mass selective detector (GC/MSD) and processed using MassWorks 6.0 post-acquisition software from Cerno Bioscience [8]. The MassWorks software suite (Version 6.0.0.0) utilizes an advanced calibration algorithm in conjunction with the built-in MSD EI tuning/calibration gas, perfluorotributylamine (PFTBA), to extend mass accuracy by correcting peak shapes to a mathematically defined function and accurately locating peak positions. In addition to achieving accurate mass correction, the continuously sampled spectral error as a function of the ion’s entire isotopic envelope is reported quantitatively as spectral accuracy. Corrected mass accuracy and spectral accuracy are used together to determine or validate elemental composition. Copies of spectra are found in the Supplementary Materials.
3.2. Preperation of Bis(1,1,1,3,3,3-hexafluoropropan-2-yl) [1,1′-biphenyl]-4,4′-dicarboxylate
To a 4-dram reaction vial with a stir bar, 4,4′-biphenyldicarboxaldehyde (4, 0.5 mmol, 105 mg, 1 equiv.), hexafluoroisopropanol (5 mmol, 263 µL 10 equiv.), pyridine (5 mmol, 404 µL, 10 equiv.), sodium persulfate (5 mmol, 1190 mg, 10 equiv.), 4-acetamido-TEMPO (3, 0.3 mmol, 64 mg, 0.60 equiv.), and acetonitrile (2 mL) were added. The contents of the vial were heated for 24 h at 50 °C in a sand bath while stirring continuously. Upon completion of the heating step, the reaction mixture was quenched with hexane (5 mL) and the vial re-capped and shaken to extract any product. The solids were allowed to settle, and the liquid decanted into a 250 mL separatory funnel. This process was repeated two further times for a total of three washes with hexane. Hexane (10 mL) was added to the separatory funnel together with deionized water (25 mL). The separatory funnel was shaken and the aqueous layer removed before adding 0.5 M hydrochloric acid (25 mL). After shaking, the aqueous layer was removed and the organic layer was dried over sodium sulfate. The hexane was removed in vacuo to afford the product, bis(1,1,1,3,3,3-hexafluoropropan-2-yl) [1,1′-biphenyl]-4,4′-dicarboxylate 5 as a white solid (126 mg, 46% yield).
MP: 102–104 °C. 1H-NMR (500 MHz, CDCl3) δ 8.27–8.21 (m, 4H), 7.80–7.74 (m, 4H), 6.05 (hept, J = 6.0 Hz, 2H). 13C-NMR (75 MHz, CDCl3) δ 162.91, 145.70, 131.16, 127.74, 126.80, 120.60 (q, J = 279.9 Hz), 67.08 (hept, J = 34.8 Hz). 19F-NMR (471 MHz, CDCl3) δ −73.06 (d, J = 6.0 Hz), GC-MS: m/z = 542, base peak of m/z = 375. IR: 2972(w), 1605(m), 1740(s) cm−1. Accurate mass (EI): m/z calculated for C17H9O3F6: 542.0382, found 542.0408 (spectral accuracy = 99.05%).
4. Conclusions
Bis-hexafluoroisopropyl ester 5 has been prepared by means of a nitroxide-catalysed oxidative functionalization reaction. Sodium persulfate was used as the terminal oxidant. Additionally, the product was isolated in a pure form by means of a simple extraction.
Supplementary Materials
Copies of the 1H-, 19F, and 13C-NMR spectra, the IR spectrum, and the GC-MS of 5.
Author Contributions
Conceptualization, N.E.L.; methodology, E.T.M. and N.E.L.; validation, E.T.M. and A.M.G.; formal analysis, E.T.M. and A.M.G.; resources, N.E.L.; data curation, E.T.M., M.S., and A.M.G.; writing—original draft preparation, E.T.M. and N.E.L.; writing—review and editing, E.T.M. and N.E.L.; supervision, M.S. and N.E.L.; project administration, N.E.L.; funding acquisition, N.E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Connecticut.
Data Availability Statement
Data are contained within the article or Supplementary Material.
Acknowledgments
We thank the University of Connecticut for the funding. We also gratefully acknowledge equipment support from Lucas Fulton (University of Connecticut) for NMR spectroscopy and Yongdong Wang (Cerno Bioscience) for providing access to MassWorks software (Version 6.0.0) for accurate GC-MS.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alho, L.D.O.G.; Gebara, R.C.; Mansano, A.D.S.; Rocha, G.S.; Melão, M.D.G.G. Individual and Combined Effects of Manganese and Chromium on a Freshwater Chlorophyceae. Environ. Toxicol. Chem. 2022, 41, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Bray, J.M.; Stephens, S.M.; Weierbach, S.M.; Vargas, K.; Lambert, K.M. Recent advancements in the use of Bobbitt’s salt and 4-acetamidoTEMPO. Chem. Commun. 2023, 59, 14063–14092. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, A.L.; Politano, F.; Witko, M.L.; Leadbeater, N.E. Preparation of Nitriles from Aldehydes Using Ammonium Persulfate by Means of a Nitroxide-Catalysed Oxidative Functionalisation Reaction. Org. Biomol. Chem. 2022, 20, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sandoval, A.L.; Leadbeater, N.E. Oxidation of Alcohols to Aldehydes and Ketones Using a Catalytic Pairing of a Nitroxide and Nitric Acid. SynOpen 2023, 7, 718–722. [Google Scholar] [CrossRef]
- Smart, B.E. Fluorine Substituent Effects (on Bioactivity). J. Fluor. Chem. 2001, 109, 3–11. [Google Scholar] [CrossRef]
- Sandoval, A.L.; Politano, F.; Witko, M.L.; Leadbeater, N.E. Preparation of Hexafluoroisopropyl Esters by Oxidative Esterification of Aldehydes Using Sodium Persulfate. Org. Biomol. Chem. 2021, 19, 2986–2990. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, M.A.; Miles, D.; Rothwell, A.P.; Wood, K.V.; Cushman, M. The “apparent” Hydrolysis of Alkyl Esters during Electrospray Ionization. Rapid Commun. Mass Spectrom. RCM 2003, 17, 1703–1708. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, M. The Concept of Spectral Accuracy for MS. Anal. Chem. 2010, 82, 7055–7062. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).