6-Amino-7-((4-methoxybenzyl)thio)quinazolin-4(3H)-one
Abstract
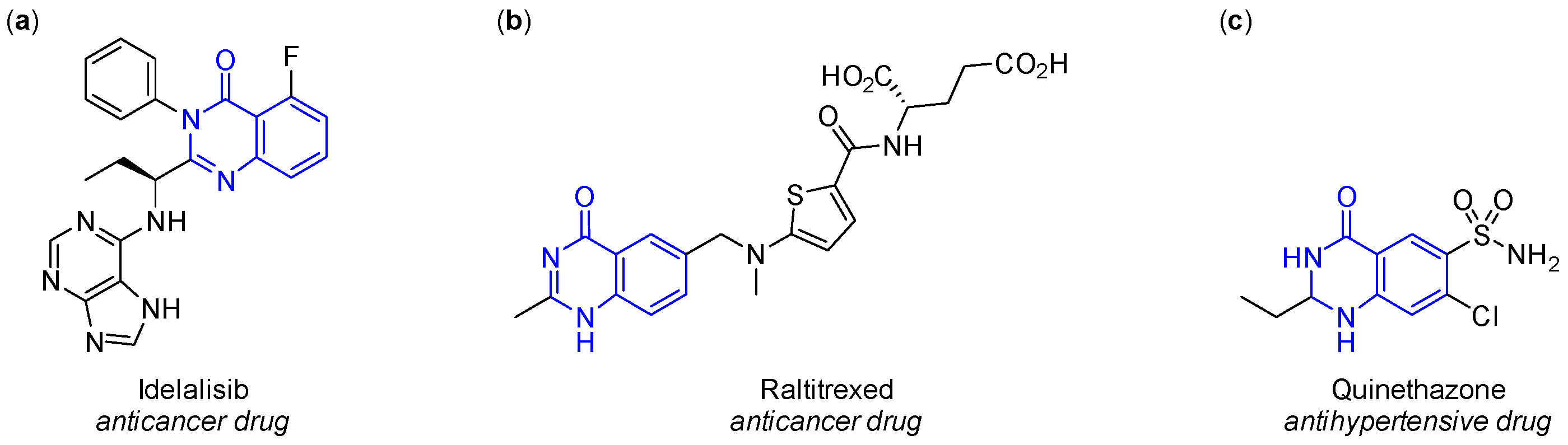
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information and Analyses
3.2. Synthesis of 7-((4-Methoxybenzyl)thio)-6-nitroquinazolin-4(3H)-one
3.3. Synthesis of 6-Amino-7-((4-methoxybenzyl)thio)quinazolin-4(3H)-one
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghoneim, M.M.; Abdelgawad, M.A.; Elkanzi, N.A.A.; Parambi, D.G.T.; Alsalahat, I.; Farouk, A.; Bakr, R.B. A Literature Review on Pharmacological Aspects, Docking Studies, and Synthetic Approaches of Quinazoline and Quinazolinone Derivatives. Arch. Der Pharm. 2024, 357, 2400057. [Google Scholar] [CrossRef] [PubMed]
- Auti, P.S.; George, G.; Paul, A.T. Recent Advances in the Pharmacological Diversification of Quinazoline/Quinazolinone Hybrids. RSC Adv. 2020, 10, 41353–41392. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Zaib, S.; Batool, S.; Abbas, N.; Ashraf, Z.; Iqbal, J.; Saeed, A. Quinazolines and Quinazolinones as Ubiquitous Structural Fragments in Medicinal Chemistry: An Update on the Development of Synthetic Methods and Pharmacological Diversification. Bioorg. Med. Chem. 2016, 24, 2361–2381. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ibrar, A.; Ahmed, W.; Saeed, A. Synthetic Approaches, Functionalization and Therapeutic Potential of Quinazoline and Quinazolinone Skeletons: The Advances Continue. Eur. J. Med. Chem. 2015, 90, 124–169. [Google Scholar] [CrossRef] [PubMed]
- Von Tresckow, J.; Heyl, N.; Robrecht, S.; Giza, A.; Aldaoud, A.; Schlag, R.; Klausmann, M.; Linde, H.; Stein, W.; Schwarzer, A.; et al. Treatment with Idelalisib in Patients with Chronic Lymphocytic Leukemia—Real World Data from the Registry of the German CLL Study Group. Ann. Hematol. 2023, 102, 3083–3090. [Google Scholar] [CrossRef]
- Soumerai, J.D.; Ni, A.; Xing, G.; Huang, J.; Furman, R.R.; Jones, J.; Sharman, J.P.; Hallek, M.; Adewoye, A.H.; Dubowy, R.; et al. Evaluation of the CLL-IPI in Relapsed and Refractory Chronic Lymphocytic Leukemia in Idelalisib Phase-3 Trials. Leuk. Lymphoma 2019, 60, 1438–1446. [Google Scholar] [CrossRef]
- Shen, Y.; Best, O.G.; Mulligan, S.P.; Christopherson, R.I. Ibrutinib and Idelalisib Block Immunophenotypic Changes Associated with the Adhesion and Activation of CLL Cells in the Tumor Microenvironment. Leuk. Lymphoma 2018, 59, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Staber, P.B.; Jurczak, W.; Greil, R.; Vucinic, V.; Middeke, J.M.; Montillo, M.; Munir, T.; Neumeister, P.; Schetelig, J.; Stilgenbauer, S.; et al. Tafasitamab Combined with Idelalisib or Venetoclax in Patients with CLL Previously Treated with a BTK Inhibitor. Leuk. Lymphoma 2021, 62, 3440–3451. [Google Scholar] [CrossRef]
- Cheng, Y.; Teng, Z.; Zhang, Y.; Jin, B.; Zheng, Z.; Man, L.; Wang, Z.; Teng, Y.; Yu, P.; Shi, J.; et al. Irinotecan plus Raltitrexed as Second-Line Treatment in Locally Advanced or Metastatic Colorectal Cancer Patients: A Prospective Open-Label, Single-Arm, Multi-Center, Phase II Study. BMC Cancer 2024, 24, 1082. [Google Scholar] [CrossRef]
- Adnan, K.; Hussain, S.; Amir, M.; Ahmed, S.; Akbar, A.; Jadoon, S.K.; Khan, S.; ZiLong, Z.; Khan, M.S.; Adnan, K.; et al. A Clinical Study of Intraoperative Perfusion Chemotherapy with Raltitrexed in Colon Cancer: A Prospective Cohort Study. Cureus 2024, 16, e58481. [Google Scholar] [CrossRef]
- Batra, A.; Rigo, R.; Hannouf, M.B.; Cheung, W.Y. Real-World Safety and Efficacy of Raltitrexed in Patients With Metastatic Colorectal Cancer. Clin. Color. Cancer 2021, 20, e75–e81. [Google Scholar] [CrossRef] [PubMed]
- Steigmann, F.; Griffin, R. Evaluation of Quinethazone, a New Diuretic. J. Am. Geriatr. Soc. 1963, 11, 945–951. [Google Scholar] [CrossRef]
- Wales, J.K. Preliminary Studies on the Action of Quinethazone in Normal Subjects and in Hypertensive Patients. Int. J. Clin. Pract. 1965, 19, 387–392. [Google Scholar] [CrossRef]
- Sperry, S.; Xiang, A.X.; Ernst, J.T.; Reich, S.H.; Sprengeler, P.A.; Shaghafi, M.; Michels, T.; Nilewski, C.; Tran, C.V.; Packard, G.K.; et al. Eif4e-Inhibiting 4-Oxo-3,4-Dihydropyrido[3,4-D]Pyrimidine Compounds 2021. Patent WO2021003157, 1 July 2021. [Google Scholar]
- Ishiguro, S.; Shimada, S.; Seya, M.; Okue, M.; Yagi, Y.; Ogane, N.; Saitou, Y. Derives D’isoquinoleine. Patent WO1998005648A1, 12 February 1998. [Google Scholar]
- Ardón-Muñoz, L.G.; Bolliger, J.L. Synthesis of Benzo[4,5]Thiazolo[2,3-c][1,2,4]Triazole Derivatives via C-H Bond Functionalization of Disulfide Intermediates. Molecules 2022, 27, 1464. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa, S.; Bolliger, J.L. 6-Amino-7-((4-methoxybenzyl)thio)quinazolin-4(3H)-one. Molbank 2024, 2024, M1942. https://doi.org/10.3390/M1942
Thapa S, Bolliger JL. 6-Amino-7-((4-methoxybenzyl)thio)quinazolin-4(3H)-one. Molbank. 2024; 2024(4):M1942. https://doi.org/10.3390/M1942
Chicago/Turabian StyleThapa, Susila, and Jeanne L. Bolliger. 2024. "6-Amino-7-((4-methoxybenzyl)thio)quinazolin-4(3H)-one" Molbank 2024, no. 4: M1942. https://doi.org/10.3390/M1942
APA StyleThapa, S., & Bolliger, J. L. (2024). 6-Amino-7-((4-methoxybenzyl)thio)quinazolin-4(3H)-one. Molbank, 2024(4), M1942. https://doi.org/10.3390/M1942






