Ethyl 4H-Pyran-4-one-2-carboxylate
Abstract
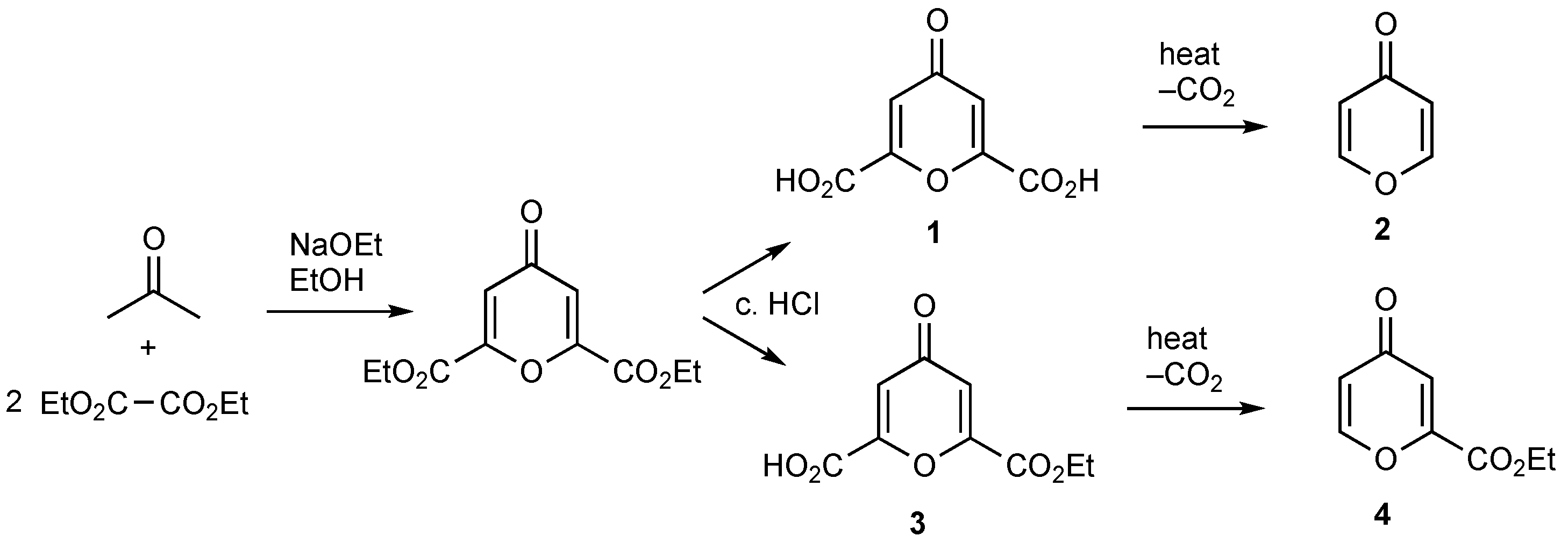
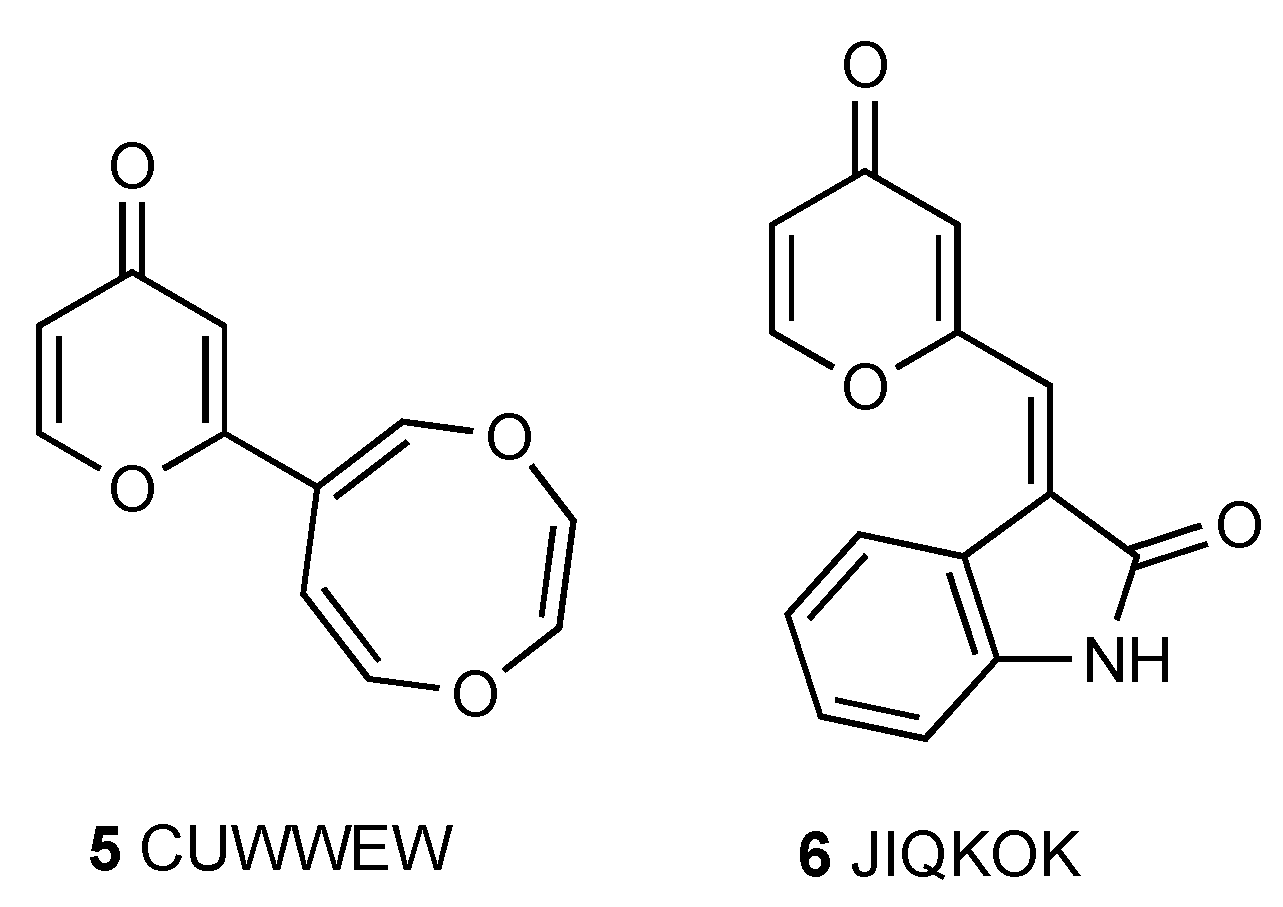
1. Introduction
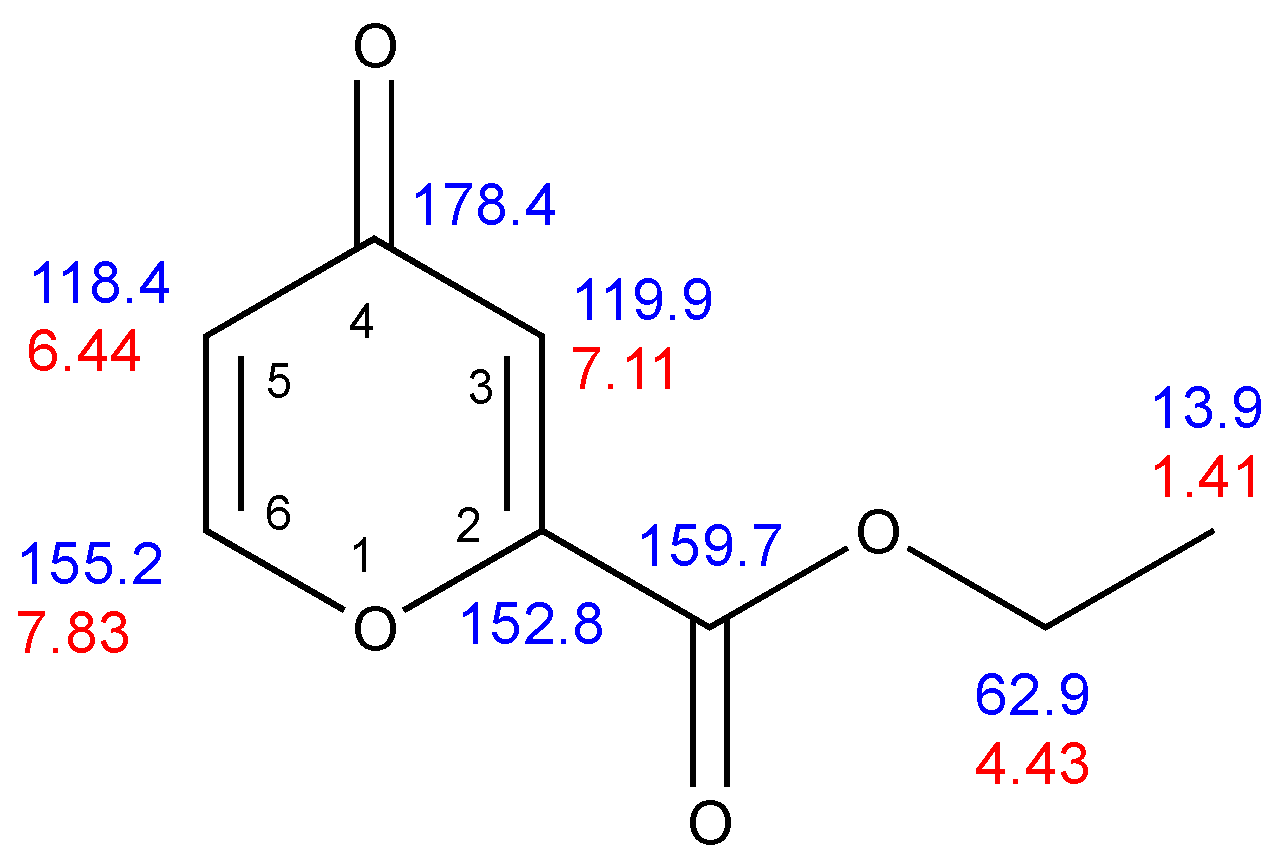
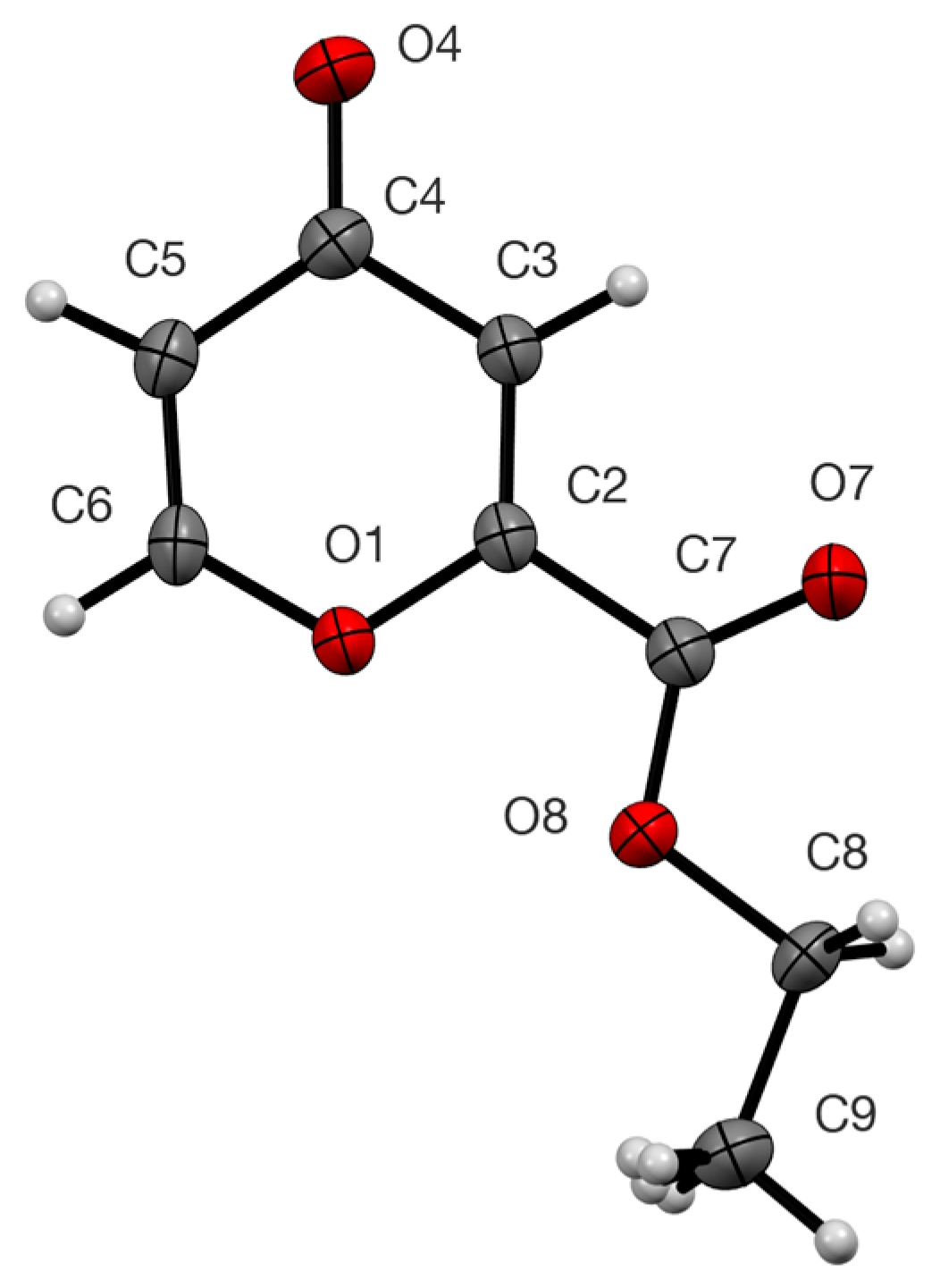
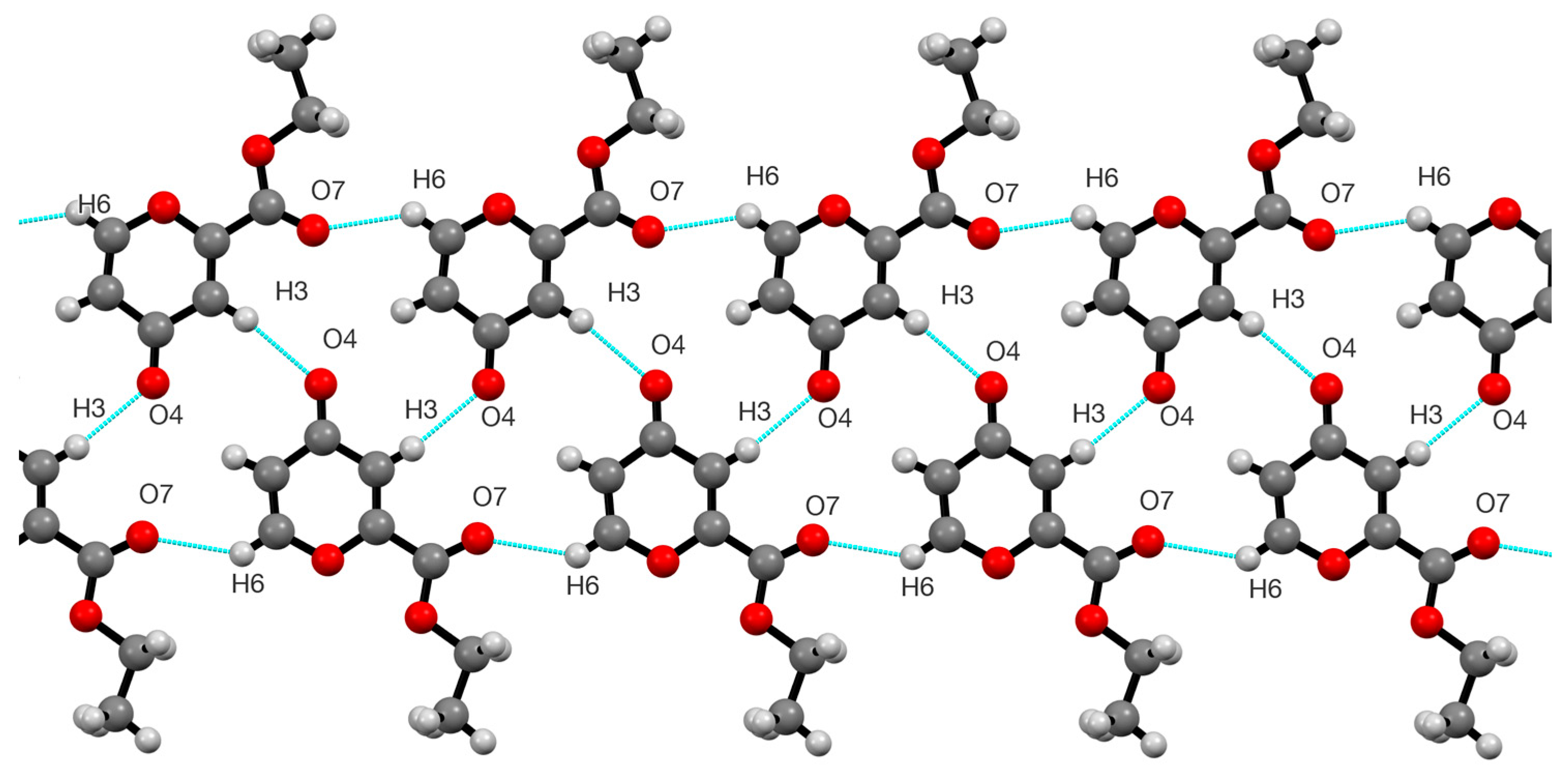
2. Results
3. Experimental Section
3.1. General Experimental Details
3.2. Formation of Ethyl 4H-Pyran-4-one-2-carboxylate 4
3.3. X-Ray Structure Determination of 4
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ost, H. Die stickstoffhaltigen Derivate der Mekonsäure. J. Prakt. Chem. 1884, 29, 57–69. [Google Scholar] [CrossRef]
- Haitinger, L.; Lieben, A. Untersuchungen über Chelidonsäure. Monatsh. Chem. 1885, 6, 279–328. [Google Scholar] [CrossRef]
- Markees, D.G. The reaction of ethyl 4H-pyran-4-one-2-carboxylate with 1,2-diaminobenzene. J. Heterocycl. Chem. 1990, 27, 1837–1838. [Google Scholar] [CrossRef]
- Lumetzberger, A.; Löwe, W.; Weber, M.; Luger, P. Synthesis of an 4-oxo-1,4-dihydropyridinomethylidene substituted 2-indolinone. J. Heterocycl. Chem. 2007, 44, 155–159. [Google Scholar] [CrossRef]
- Obydennov, D.L.; Steben’kov, V.D.; Obydennov, K.L.; Usachev, S.A.; Moshkin, V.S.; Sosnovskikh, V.Y. Reactions of 4-pyrones with azomethine ylides as a chemoselective method for the construction of multisubstituted pyrano[2,3-c]pyrrolidines. Synthesis 2021, 53, 2621–2631. [Google Scholar] [CrossRef]
- Elvidge, J.A.; Stevens, R. Acylcyclopentanetriones: Comments in a recent synthesis and observations from proton resonance on their enolisation and on isomerism in 5-methoxyhept-4-en-3-one. J. Chem. Soc. 1965, 2251–2257. [Google Scholar] [CrossRef]
- Huke, J.P.; Hillier, I.H. The electronic structure of chromones studied by low-energy photoelectron spectroscopy and ab initio molecular orbital calculations. J. Chem. Soc. Perkin Trans. 1985, 2, 1191–1194. [Google Scholar] [CrossRef]
- Palmer, M.H.; Coreno, M.; de Simone, M.; Grazioli, C.; Jones, N.C.; Hoffmann, S.V.; Aitken, R.A.; Sonecha, D.K. The ionic and ground states of gamma-pyrone. The photoionization spectrum studied by synchrotron radiation and interpreted by configuration interaction and density functional calculations. J. Chem. Phys. 2023, 158, 014304. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.H.; Hoffmann, S.V.; Jones, N.C.; Coreno, M.; de Simone, M.; Grazioli, C.; Aitken, R.A.; Perrault, L.; Patterson, I.L.J. The ultraviolet and vacuum ultraviolet absorption spectrum of gamma-pyrone; the singlet states studied by configuration interaction and density functional calculations. J. Chem. Phys. 2024, 160, 054305. [Google Scholar] [CrossRef]
- Riegel, E.R.; Zwilgmeyer, F. Chelidonic acid. Org. Synth. 1937, 17, 40–41. [Google Scholar] [CrossRef]
- De Souza, C.; Hajikarimian, Y.; Sheldrake, P.W. A convenient method for the preparation of pyran-4-one. Synth. Commun. 1992, 22, 755–759. [Google Scholar] [CrossRef]
- Hansen, P.E. Carbon-hydrogen spin-spin coupling constants. Prog. Nucl. Magn. Reson. Spectrosc. 1981, 14, 175–295. [Google Scholar] [CrossRef]
- Mayo, R.E.; Goldstein, J.H. Proton magnetic resonance and 13C–H satellite spectra of 4-pyrone: Analysis and assignments. Spectrochim. Acta A 1967, 23, 55–60. [Google Scholar] [CrossRef]
- Kingsbury, C.A.; Cliffton, M.; Looker, J.H. Carbon-13 nuclear magnetic resonance spectra of kojic acid and other 4-pyrone derivatives. J. Org. Chem. 1976, 41, 2777–2786. [Google Scholar] [CrossRef]
- Altenbach, H.-J.; Lex, J.; Linkenheil, D.; Voss, B.; Vogel, E. Synthesis of the antibiotic LL-Z1120—Contribution to an understanding of the 1,4-dioxocine system. Angew. Chem. Int. Ed. 1984, 23, 966–968. [Google Scholar] [CrossRef]
- Attenburrow, J.; Elks, J.; Elliott, D.F.; Hems, B.A.; Harris, J.O.; Brodrick, C.I. Experiments relating to the synthesis of patulin Part 1. A study of hydrogenated γ-pyrones. J. Chem. Soc. 1945, 571–577. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
| Position | |||||||
|---|---|---|---|---|---|---|---|
| δC | δH | 3JH–H | 4JH–H | 1JC–H | 2JC–H | 3JC–H | |
| 2 | 152.8 | – | – | 8.4 | 4.0 | ||
| 3 | 119.9 | 7.11 | 2.4 | 172.3 | – | 3.8 | |
| 4 | 178.4 | – | – | 7.1, 1.5 | 1.5 | ||
| 5 | 118.4 | 6.44 | 5.7 | 2.4 | 169.8 | 7.8 | 3.4 |
| 6 | 155.2 | 7.83 | 5.7 | 199.7 | 7.2 | – | |
| Ester CO | 159.7 | – | – | 3.3, 3.3 | |||
| CH2 | 62.9 | 4.43 | 7.2 | 149.2 | 4.45 | – | |
| CH3 | 13.9 | 1.41 | 7.2 | 127.5 | 2.6 | – |
| Bond Lengths | Internal Angles | ||
|---|---|---|---|
| O(1)–C(2) | 1.3629(17) | C(6)–O(1)–C(2) | 117.02(11) |
| C(2)–C(3) | 1.335(2) | O(1)–C(2)–C(3) | 123.68(13) |
| C(3)–C(4) | 1.458(2) | C(2)–C(3)–C(4) | 120.75(14) |
| C(4)–O(4) | 1.2339(19) | C(3)–C(4)–C(5) | 113.93(13) |
| C(4)–C(5) | 1.452(2) | C(4)–C(5)–C(6) | 120.45(14) |
| C(5)–C(6) | 1.332(2) | C(5)–C(6)–O(1) | 124.18(13) |
| C(6)–O(1) | 1.3653(18) |
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| C(3)–H(3)···O(4) | 0.950 | 2.309 | 3.249 | 169.95 |
| C(6)–H(6)···O(7) | 0.950 | 2.351 | 3.116 | 137.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.A.; Nelson, A.J.B.; Slawin, A.M.Z. Ethyl 4H-Pyran-4-one-2-carboxylate. Molbank 2024, 2024, M1939. https://doi.org/10.3390/M1939
Aitken RA, Nelson AJB, Slawin AMZ. Ethyl 4H-Pyran-4-one-2-carboxylate. Molbank. 2024; 2024(4):M1939. https://doi.org/10.3390/M1939
Chicago/Turabian StyleAitken, R. Alan, Alexander J. B. Nelson, and Alexandra M. Z. Slawin. 2024. "Ethyl 4H-Pyran-4-one-2-carboxylate" Molbank 2024, no. 4: M1939. https://doi.org/10.3390/M1939
APA StyleAitken, R. A., Nelson, A. J. B., & Slawin, A. M. Z. (2024). Ethyl 4H-Pyran-4-one-2-carboxylate. Molbank, 2024(4), M1939. https://doi.org/10.3390/M1939







