3-Methyl-2-(5-((trimethylsilyl)ethynyl)pyridin-2-yl)butan-2-ol
Abstract
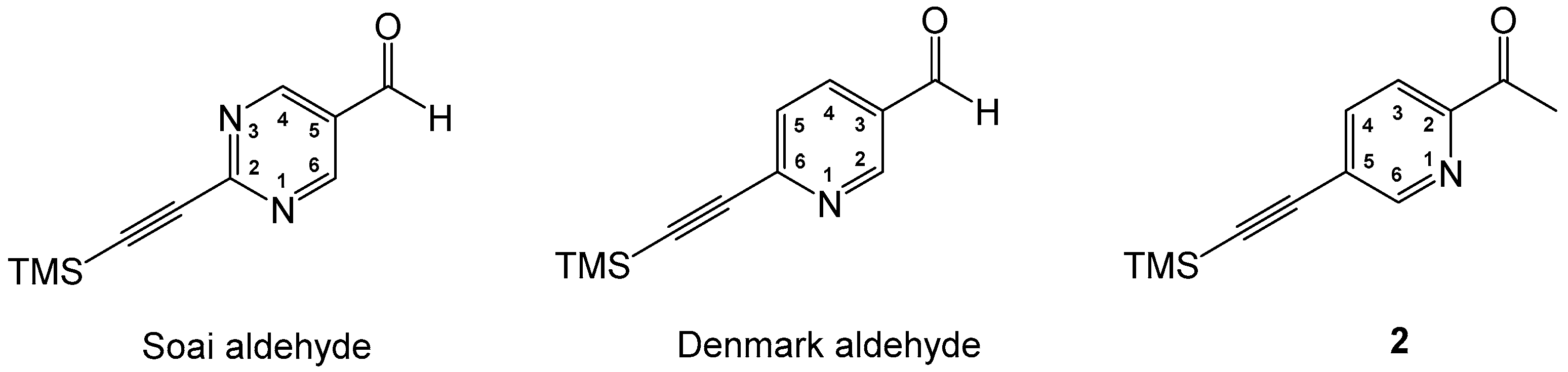
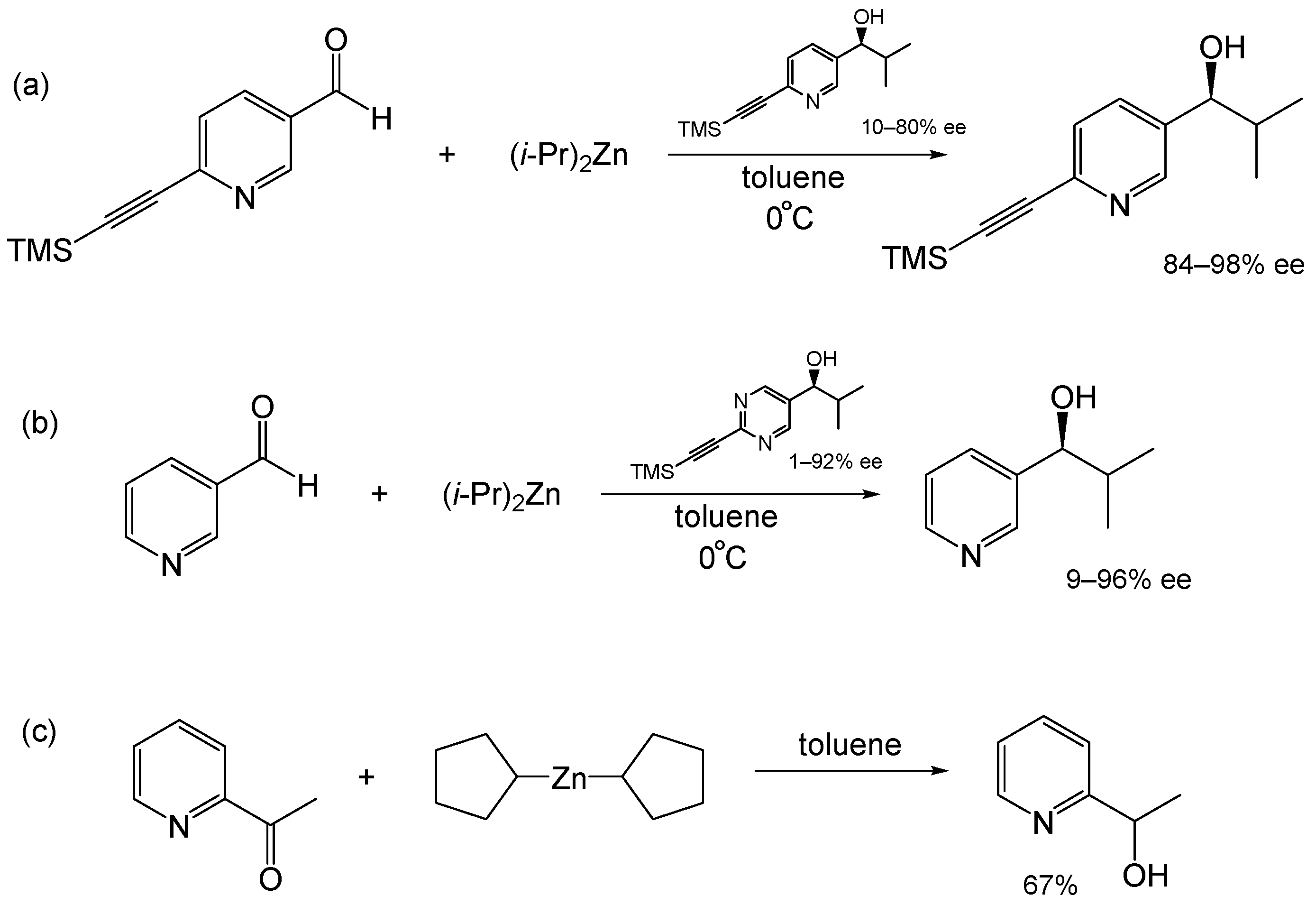
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Chemical Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carruthers, W.; Coldham, I. Modern Methods of Organic Synthesis; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Bauer, T. Enantioselective dialkylzinc-mediated alkynylation, arylation and alkenylation of carbonyl groups. Coord. Chem. Rev. 2015, 299, 83–150. [Google Scholar] [CrossRef]
- Pellissier, H. Recent developments in enantioselective zinc-catalyzed transformations. Coord. Chem. Rev. 2021, 439, 213926. [Google Scholar] [CrossRef]
- Dimitrov, V.; Kamenova-Nacheva, M. Enantioselective organozinc-catalyzed additions to carbonyl compounds—Recent developments. J. Chem. Technol. Metall. 2009, 44, 317–332. [Google Scholar]
- Pu, L.; Yu, H.B. Catalytic asymmetric organozinc additions to carbonyl compounds. Chem. Rev. 2001, 101, 757–824. [Google Scholar] [CrossRef] [PubMed]
- Soai, K.; Shibata, T.; Morioka, H.; Choji, K. Asymmetric autocatalysis and amplification of enantiomeric excess of a chiral molecule. Nature 1995, 378, 767–768. [Google Scholar] [CrossRef]
- Shibata, T.; Morioka, H.; Hayase, T.; Choji, K.; Soai, K. Highly enantioselective catalytic asymmetric automultiplication of chiral pyrimidyl alcohol. J. Am. Chem. Soc. 1996, 118, 471–472. [Google Scholar] [CrossRef]
- Athavale, S.V.; Simon, A.; Houk, K.N.; Denmark, S.E. Structural contributions to autocatalysis and asymmetric amplification in the Soai reaction. J. Am. Chem. Soc. 2020, 142, 18387–18406. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M. Reaction mechanism in the study of amplifying asymmetric autocatalysis. In Asymmetric Autocatalysis: The Soai Reaction; Soai, K., Kawasaki, T., Matsumoto, A., Eds.; Royal Society of Chemistry: Cambridge, UK, 2022; Volume 43, pp. 97–128. [Google Scholar]
- Folkertsma, E.; Benthem, S.H.; Jastrzebski, J.T.B.H.; Lutz, M.; Moret, M.-E.; Gebbink, R.J.M.K. 1,2-Addition of diethylzinc to a bis(imidazolyl)ketone ligand. Eur. J. Inorg. Chem. 2018, 2018, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Knochel, P.; Singer, R.D. Preparation and reactions of polyfunctional organozinc reagents in organic synthesis. Chem. Rev. 1993, 93, 2117–2188. [Google Scholar] [CrossRef]
- Knochel, P.; Millot, N.; Rodriguez, A.L.; Tucker, C.E. Preparation and applications of functionalized organozinc compounds. Org. React. 2004, 58, 417–759. [Google Scholar]
- Rathke, M.W. The Reformatsky reaction. Org. React. 1975, 22, 423–460. [Google Scholar]
- Chinchilla, R.; Nájera, C. The Sonogashira reaction: A booming methodology in synthetic organic chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef] [PubMed]
- Sato, I.; Urabe, H.; Ishii, S.; Tanji, S.; Soai, K. Asymmetric synthesis with a chiral catalyst generated from asymmetric autocatalysis. Org. Lett. 2001, 3, 3851–3854. [Google Scholar] [CrossRef] [PubMed]
- Saigitbatalova, E.S.; Latypova, L.Z.; Zagidullin, A.A.; Kurbangalieva, A.R.; Gridnev, I.D. The reduction of carbonyl compounds with dicyclopentylzinc: A new example of asymmetric amplifying autocatalysis. Int. J. Mol. Sci. 2023, 24, 17048. [Google Scholar] [CrossRef] [PubMed]
- Zong, R.; Wang, D.; Hammitt, R.; Thummel, R.P. Synthetic approaches to polypyridyl bridging ligands with proximal multidentate binding sites. J. Org. Chem. 2006, 71, 167–175. [Google Scholar] [CrossRef] [PubMed]
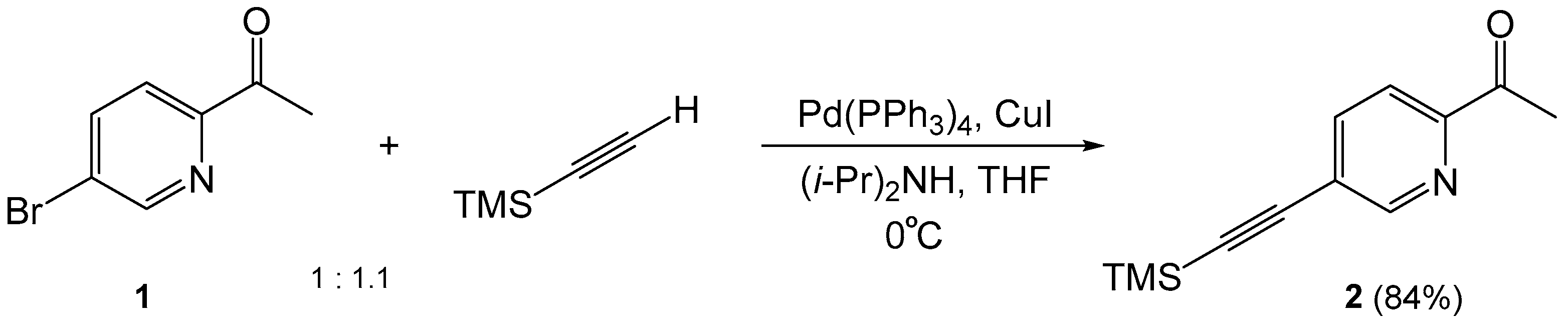

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailov, O.A.; Kurbangalieva, A.R.; Gridnev, I.D. 3-Methyl-2-(5-((trimethylsilyl)ethynyl)pyridin-2-yl)butan-2-ol. Molbank 2024, 2024, M1925. https://doi.org/10.3390/M1925
Mikhailov OA, Kurbangalieva AR, Gridnev ID. 3-Methyl-2-(5-((trimethylsilyl)ethynyl)pyridin-2-yl)butan-2-ol. Molbank. 2024; 2024(4):M1925. https://doi.org/10.3390/M1925
Chicago/Turabian StyleMikhailov, Oleg A., Almira R. Kurbangalieva, and Ilya D. Gridnev. 2024. "3-Methyl-2-(5-((trimethylsilyl)ethynyl)pyridin-2-yl)butan-2-ol" Molbank 2024, no. 4: M1925. https://doi.org/10.3390/M1925
APA StyleMikhailov, O. A., Kurbangalieva, A. R., & Gridnev, I. D. (2024). 3-Methyl-2-(5-((trimethylsilyl)ethynyl)pyridin-2-yl)butan-2-ol. Molbank, 2024(4), M1925. https://doi.org/10.3390/M1925






