4-(10-Phenyl-9-Anthracenyl)-1,2-Benzenediol
Abstract
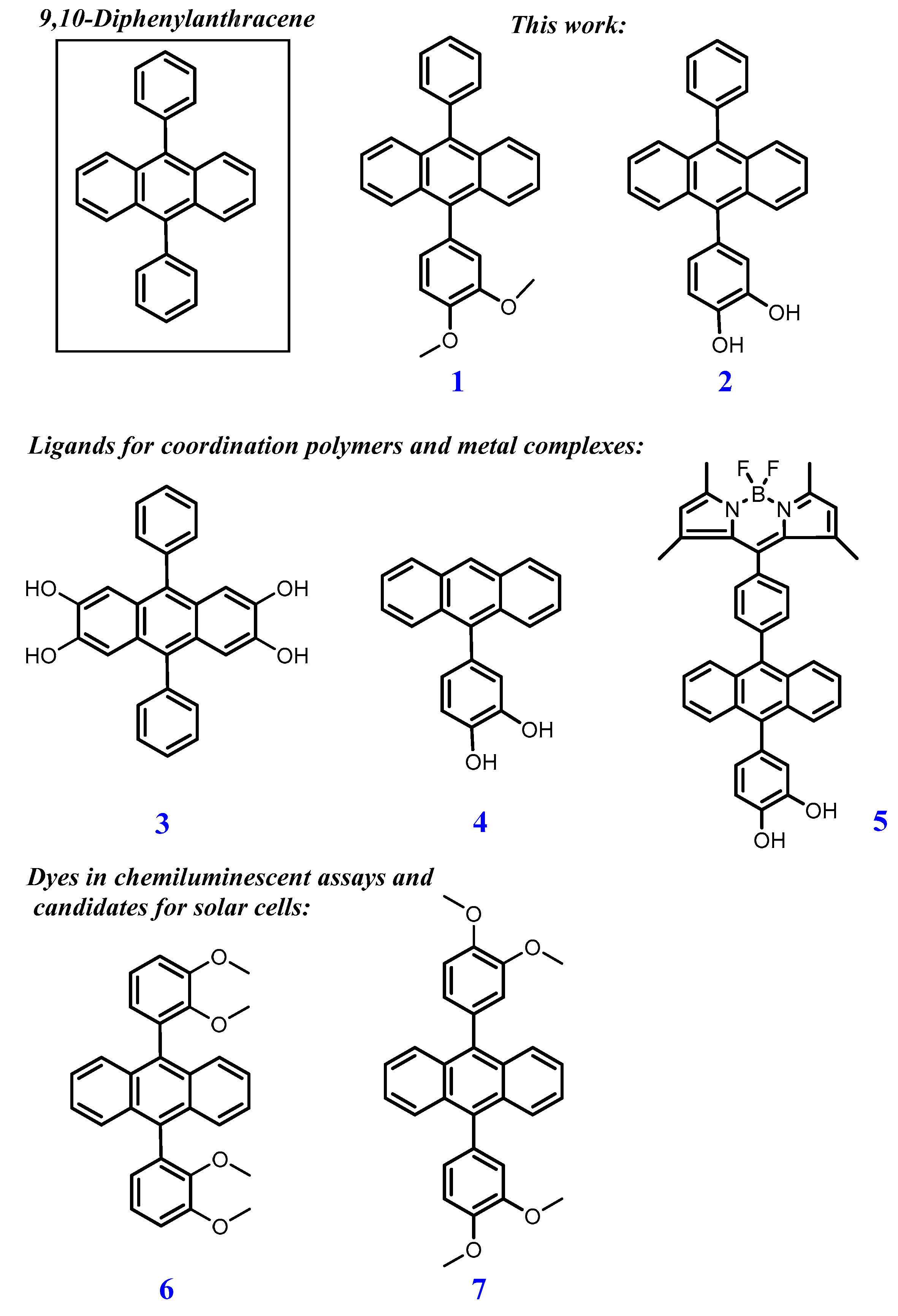
1. Introduction
2. Results and Discussion
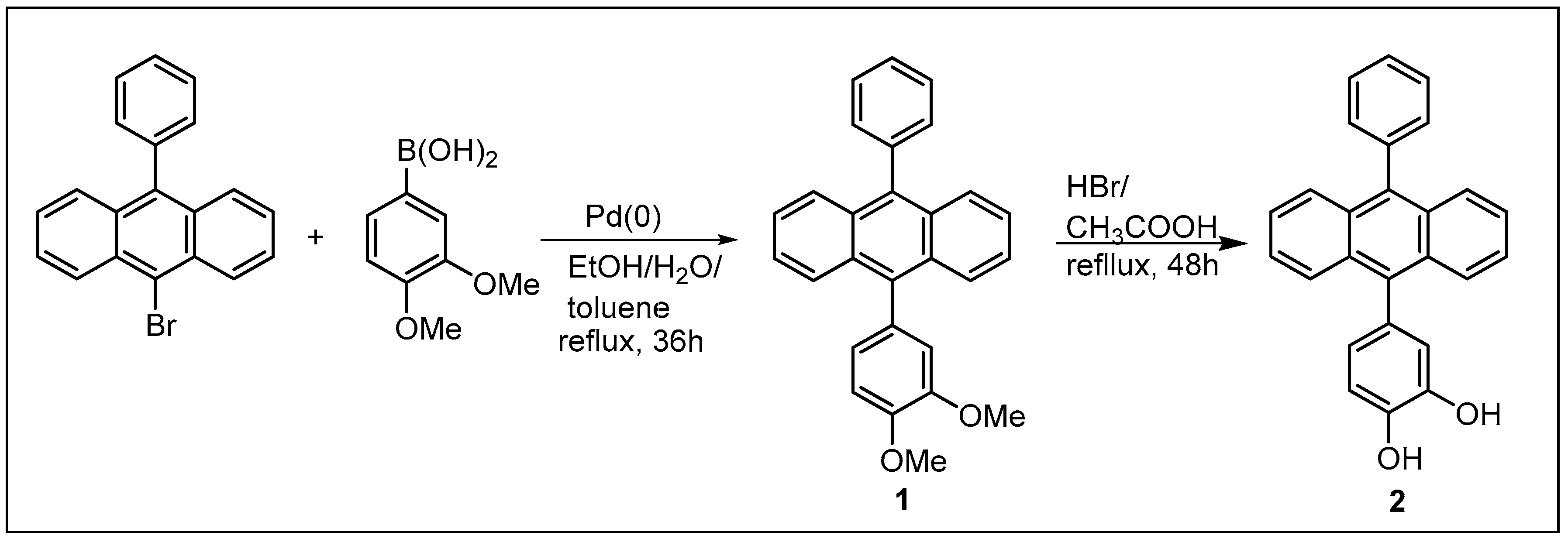
2.1. Synthesis
2.2. Characterization
2.2.1. Analysis of Compound 1
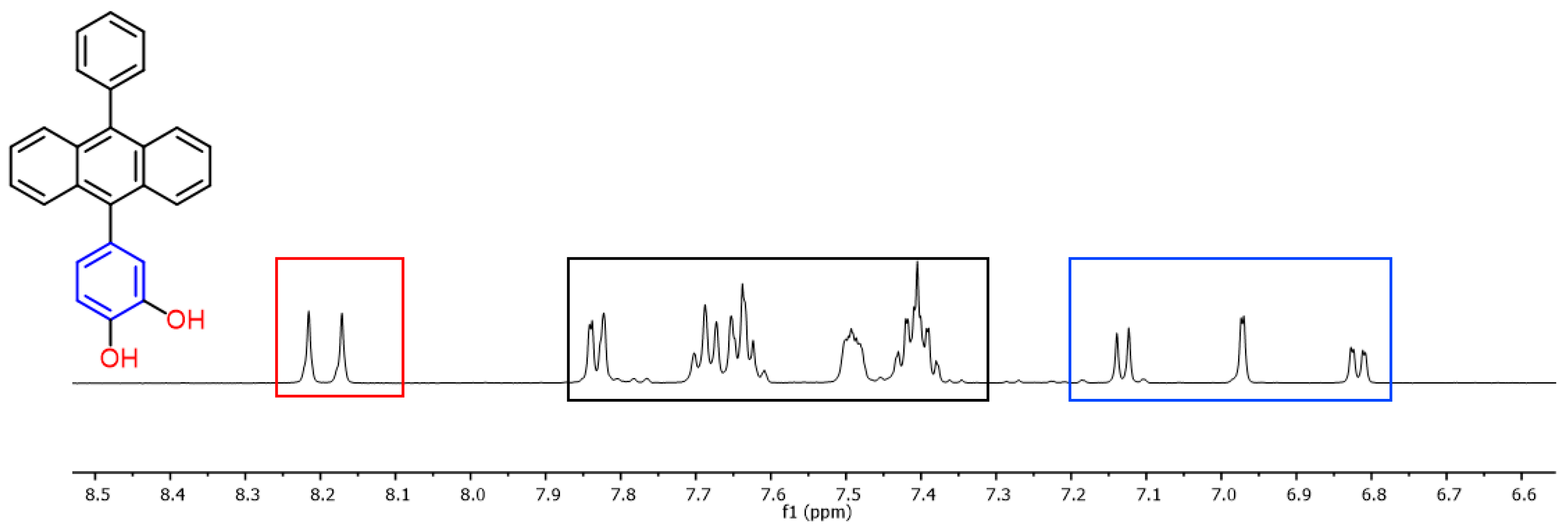
2.2.2. Analysis of Compound 2
2.3. Photophysical Studies
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Babu, S.; Hollamby, M.; Aimi, J.; Ozawa, H.; Saeki, A.; Seki, S.; Kobayashi, K.; Hagiwara, K.; Yoshizawa, M.; Möhwald, H.; et al. Nonvolatile liquid anthracenes for facile full-colour luminescence tuning at single blue-light excitation. Nat. Commun. 2013, 4, 1969. [Google Scholar] [CrossRef] [PubMed]
- Brosamer, K.; Kourentzi, K.; Willson, R.C.; Vu, B.V. Glowstick-inspired smartphone-readable reporters for sensitive, multiplexed lateral flow immunoassays. Commun. Eng. 2023, 2, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Make Glow Sticks—The Science. Available online: https://www.instructables.com/Make-Glow-Sticks-The-Science/ (accessed on 18 August 2024).
- Lo, C.-T.; Abiko, Y.; Kosai, J.; Watanabe, Y.; Nakabayashi, K.; Mori, H. Synthesis and Optoelectronic Properties of Block and Random Copolymers Containing Pendant Carbazole and (Di)phenylanthracene. Polymers 2018, 10, 721. [Google Scholar] [CrossRef]
- Kim, A.; Lee, J.; Kim, B.G.; Lee, D.J.; Jung, H. 9,10-Diphenylanthracene immobilized in mesostructured silica materials with a highly efficient deep-blue fluorescent property. J. Lumin. 2017, 190, 154–160. [Google Scholar] [CrossRef]
- Wang, D.; Hu, W.; Reinhart, B.J.; Zhang, X.; Huang, J. Tuning the Charge Transport Property and Photocatalytic Activity of Anthracene-Based 1D π–d Conjugated Coordination Polymers by Interlayer Stacking. ACS Appl. Mater. Interfaces 2022, 14, 42171–42177. [Google Scholar] [CrossRef]
- Katayama, K.; Hirotsu, M.; Kinoshita, I.; Teki, Y. Synthesis, magnetic properties and dynamic behavior of cobalt complexes with an anthracene-containing dioxolene ligand. Dalt. Trans. 2014, 43, 13384. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Hirotsu, M.; Ito, A.; Teki, Y. Fluorescence behaviour of an anthracene–BODIPY system affected by spin states of a dioxolene–cobalt centre. Dalt. Trans. 2016, 45, 10165–10172. [Google Scholar] [CrossRef] [PubMed]
- Belghiti, N.; Bennani, M.; Hamidi, M.; Bouzzine, S.M.; Bouachrine, M. New compounds based on anthracene as a good candidate for organic dye-sensitized solar cells: Theoretical investigations. Afr. J. Pure Appl. Chem. 2012, 6, 164–172. [Google Scholar] [CrossRef]
- Lopes, C.C.; Lopes, R.S.C.; Chantre, L.G.F. Processo de Síntese do Luminol, kit para a Detecção de Resíduos de Sangue Oculto e Seus Usos. Brazil Patent BR 102014014163-4-A2, 6 October 2014. [Google Scholar]
- Cammidge, A.N.; King, A.S.H. Model studies towards liquid crystalline dendrimers with mesogenic repeat units throughout the structure. Tetrahedron Lett. 2006, 47, 5569–5572. [Google Scholar] [CrossRef]
- Wuts, P.G.M. Greene’s Protective Groups in Organic Synthesis, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 29–30. ISBN 978-1-118-05748-3. [Google Scholar]
- Morris, J.V.; Mahaney, M.A.; Huber, J.R. Fluorescence quantum yield determinations. 9,10-Diphenylanthracene as a reference standard in different solvents. J. Phys. Chem. 1976, 80, 969–974. [Google Scholar] [CrossRef]
- Zhang, X.M.; Bordwell, F.G.; Bares, J.E.; Cheng, J.P.; Petrie, B.C. Homolytic bond dissociation energies of the acidic carbon-hydrogen bonds in alpha.-substituted and 10-substituted 9-methylanthracenes and their related radical anions. J. Org. Chem. 1993, 58, 3051–3059. [Google Scholar] [CrossRef]
- Recording Fluorescence Quantum Yields. Available online: http://www.horiba.com/fileadmin/uploads/Scientific/Documents/Fluorescence/quantumyieldstrad.pdf (accessed on 12 August 2024).

| Compound | Absorption | Emission | ||
|---|---|---|---|---|
| λmax (nm) (log ε a) | λmax (nm) | Stokes’ Shift | Φ (%) | |
| DPA b | 259 (5.0), 338 (3.6), 354 (3.80), 374 (4.04), 394 (4.00) | 408, 438 | 15 | 95 c |
| 1 | 259 (5.35), 338 (3.92), 355 (4.24), 374 (4.45), 394 (4.43) | 434 | 40 | 70 |
| 2 | 259 (5.47), 338 (4.00), 355 (4.32), 374 (4.53), 394 (4.51) | 437 | 43 | 63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edwards, N.; Harris, K. 4-(10-Phenyl-9-Anthracenyl)-1,2-Benzenediol. Molbank 2024, 2024, M1884. https://doi.org/10.3390/M1884
Edwards N, Harris K. 4-(10-Phenyl-9-Anthracenyl)-1,2-Benzenediol. Molbank. 2024; 2024(3):M1884. https://doi.org/10.3390/M1884
Chicago/Turabian StyleEdwards, Nicola, and Kelsey Harris. 2024. "4-(10-Phenyl-9-Anthracenyl)-1,2-Benzenediol" Molbank 2024, no. 3: M1884. https://doi.org/10.3390/M1884
APA StyleEdwards, N., & Harris, K. (2024). 4-(10-Phenyl-9-Anthracenyl)-1,2-Benzenediol. Molbank, 2024(3), M1884. https://doi.org/10.3390/M1884






