2-Methylpropan-2-ammonium [(3R,5R)-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-[(2S)-2-methylbutanoyl]oxy-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]-3,5-heptanoate
Abstract
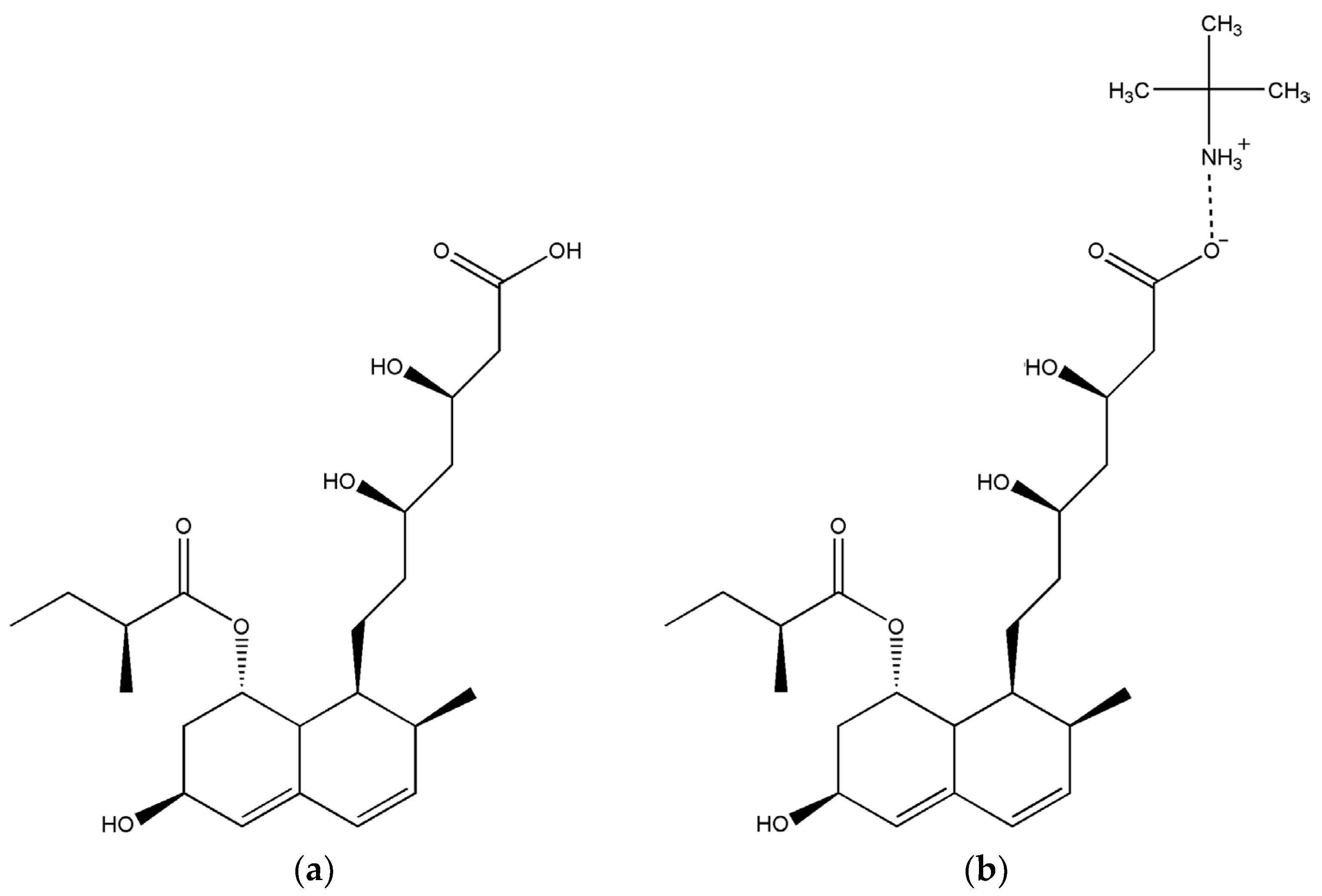
1. Introduction
2. Results
2.1. X-ray Structure Analysis of Prav-tbua
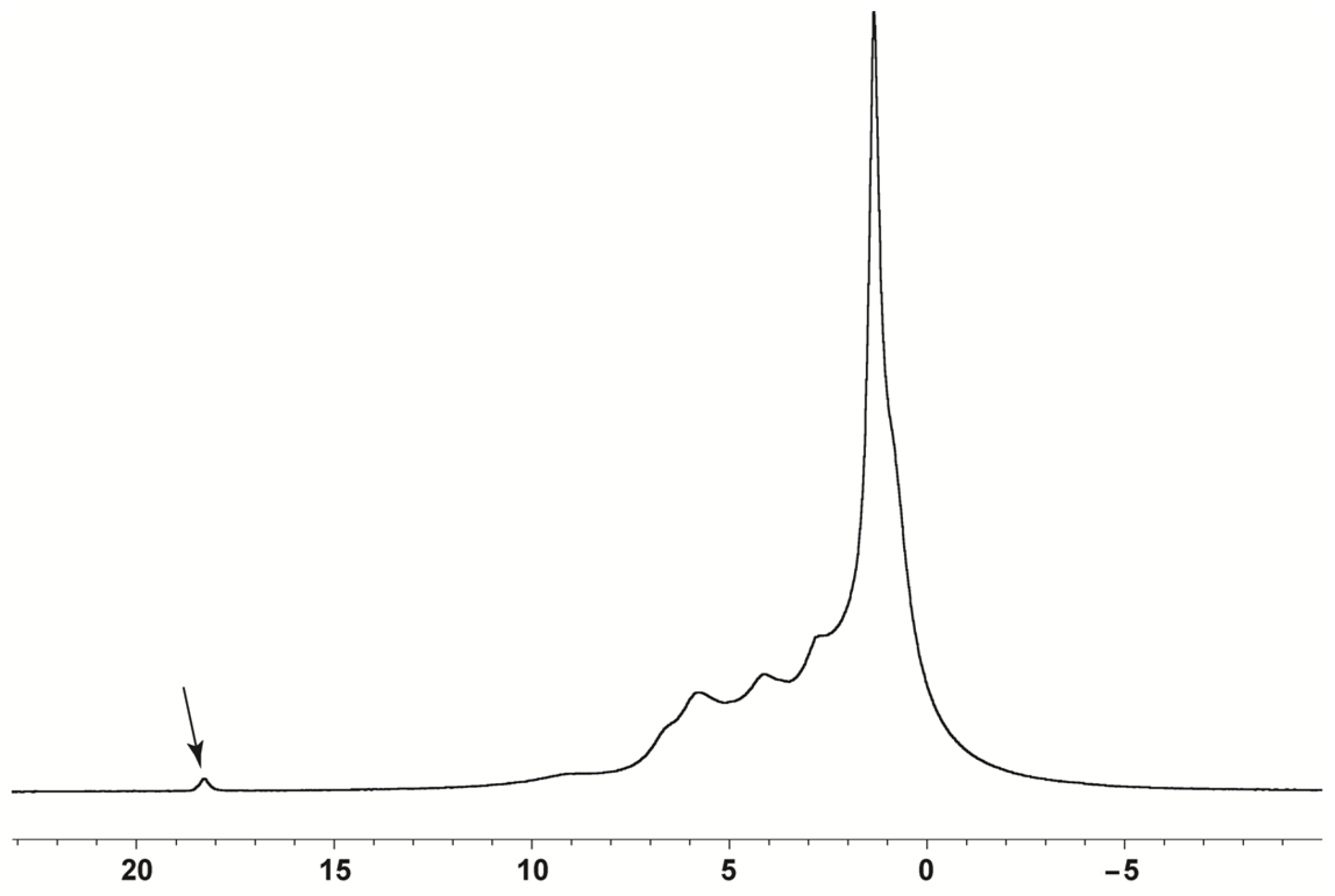
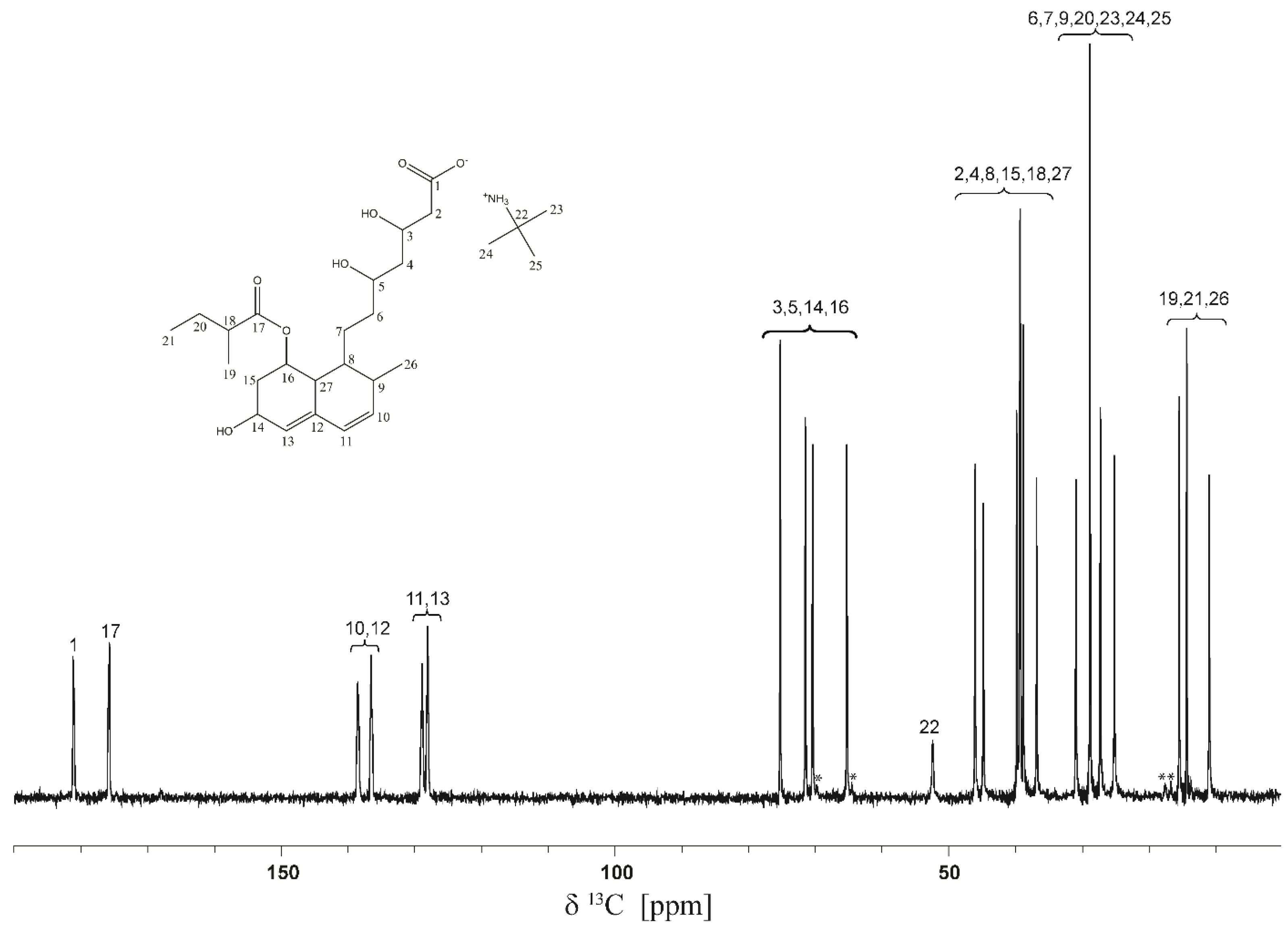
2.2. Solid-State NMR Analysis
3. Materials and Methods
3.1. General and Sample Preparations
3.2. X-ray Diffraction and Structure Refinement
3.3. 13C Solid-State NMR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Taylor, F.; Huffman, M.D.; Macedo, A.F.; Moore, T.H.M.; Burke, M.; Smith, G.D.; Ward, K.; Ebrahim, S.; Gay, H.C. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013, 2013, CD004816. [Google Scholar] [CrossRef] [PubMed]
- Nanovskaya, T.N.; Patrikeeva, S.L.; Paul, J.; Constantine, M.; Hakins, G.D.V.; Ahmed, M.S. Transplacentar Transfer and Distribution of Pravastatin. Am. J. Obstet. Gynecol. 2013, 209, 373.e1–373.e5. [Google Scholar] [CrossRef] [PubMed]
- Del Sol, A.I.; Nanayakkara, P.W.B. Pravastatin: An evidence-based statin? Expert Opin. Drug Metab. Toxicol. 2008, 4, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yamaoka, O.; Uchida, K.; Morigami, N.; Sugimoto, Y.; Fujita, T.; Inoue, T.; Fuchi, T.; Hachisuka, M.; Ueshima, H.; et al. Pravastatin reduces restenosis after coronary angioplasty of high grade stenotic lesions: Results of SHIPS (SHIga Pravastatin Study). Cardiovasc. Drug Ther. 1996, 10, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Valenti, S.; Barrio, M.; Negrier, P.; Romanini, M.; Macovez, R.; Tamarit, J.L. Comparative physical Study of Three pharmaceutically Active Benzodiazepine Derivatives: Crystalline versus Amorphous State and Crystallization Tendency. Mol. Pharm. 2021, 18, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Serajuddin, A.T. Salt formation to improve drug solubility. Adv. Drug Deliv. Rev. 2007, 59, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.G.; Kumar, A.; Gide, P.S. Formulation of solid self-nanoemulsifying drug delivery systems using N-methyl pyrrolidone as cosolvent. Drug Dev. Ind. Pharm. 2015, 41, 1460–1463. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins. 1. Drug Solubilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef]
- Turza, A.; Borodi, G.; Muresan-Pop, M.; Ulici, A. Polymorphism and β-cyclodextrin complexation of methyldrostanolone. J. Mol. Struct. 2022, 1250, 131852. [Google Scholar] [CrossRef]
- Turza, A.; Ulici, A.; Muresan-Pop, M.; Borodi, G. Solid forms and β-cyclodextrin complexation of turinabol. Acta Cryst. C 2022, C78, 305–313. [Google Scholar] [CrossRef]
- Borodi, G.; Miclaus, M.O.; Muresan-Pop, M.; Turza, A. Solid Forms and β-cyclodextrin complexation of Oxymetholone and Crystal Structure of Metribolone. Crystals 2024, 14, 483. [Google Scholar] [CrossRef]
- Porter, C.J.; Trevaskis, N.L.; Charman, W.N. Lipid and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Borodi, G.; Turza, A.; Onija, O.; Bende, A. Succinic, fumaric, adipic and oxalic acid cocrystals of promethazine hydrochloride. Acta Cryst. Sect. C Struct. Chem. 2019, 75, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Kanaujia, P.; Poovizhi, P.; Ng, W.K.; Tan, R.B.H. Amorphous formulations for dissolution and bioavailability enhancement of poorly soluble APIs. Powder Technol. 2015, 285, 2–15. [Google Scholar] [CrossRef]
- Sato, S.; Furukawa, Y. X-ray crystal structure of the tert-octyamine salt (RMS-431) of pravastatin. J. Antibiot. 1988, 41, 1265. [Google Scholar] [CrossRef] [PubMed]
- Bis, J.A.; Zaworotko, M.J. The 2-Aminopyridinium-carboxylate Supramolecular Heterosynthon: A Robust Motif for Generation of Multiple-Component Crystals. Cryst. Growth Des. 2005, 5, 1169–1179. [Google Scholar] [CrossRef]
- CrysAlis PRO. Rigaku Oxford Diffraction; CrysAlis PRO: Yarnton, UK, 2015. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. SUPERFLIP-a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]

| Identification Code | Prav-tbua |
|---|---|
| Empirical formula | C27H47NO7 |
| Formula weight | 497.65 |
| Temperature/K | 293(2) |
| Crystal system | orthorhombic |
| Space group | P212121 |
| a/Å | 5.98809(13) |
| b/Å | 17.4955(3) |
| c/Å | 28.0358(6) |
| α/° | 90 |
| β/° | 90 |
| γ/° | 90 |
| Volume/Å3 | 2937.17(10) |
| Z | 4 |
| ρcalc g/cm3 | 1.125 |
| μ/mm−1 | 0.648 |
| F(000) | 1088.0 |
| Radiation | CuKα (λ = 1.54184) |
| 2Θ range/° | 6.306 to 143.05 |
| Index ranges | −7 ≤ h ≤ 7, −19 ≤ k ≤ 21, −25 ≤ l ≤ 33 |
| Reflections collected | 12,408 |
| Independent reflections | 5198 [Rint = 0.0350, Rsigma = 0.0643] |
| Data/restraints/parameters | 5198/1/329 |
| Goodness-of-fit on F2 | 1.061 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0646, wR2 = 0.1716 |
| Final R indexes [all data] | R1 = 0.1060, wR2 = 0.1890 |
| Largest diff. peak/hole/e Å−3 | 0.29/−0.18 |
| Flack parameter | 0.12(11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosu, I.-G.; Turza, A.; Filip, X.; Miclaus, M.-O. 2-Methylpropan-2-ammonium [(3R,5R)-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-[(2S)-2-methylbutanoyl]oxy-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]-3,5-heptanoate. Molbank 2024, 2024, M1885. https://doi.org/10.3390/M1885
Grosu I-G, Turza A, Filip X, Miclaus M-O. 2-Methylpropan-2-ammonium [(3R,5R)-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-[(2S)-2-methylbutanoyl]oxy-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]-3,5-heptanoate. Molbank. 2024; 2024(3):M1885. https://doi.org/10.3390/M1885
Chicago/Turabian StyleGrosu, Ioana-Georgeta, Alexandru Turza, Xenia Filip, and Maria-Olimpia Miclaus. 2024. "2-Methylpropan-2-ammonium [(3R,5R)-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-[(2S)-2-methylbutanoyl]oxy-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]-3,5-heptanoate" Molbank 2024, no. 3: M1885. https://doi.org/10.3390/M1885
APA StyleGrosu, I.-G., Turza, A., Filip, X., & Miclaus, M.-O. (2024). 2-Methylpropan-2-ammonium [(3R,5R)-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-[(2S)-2-methylbutanoyl]oxy-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]-3,5-heptanoate. Molbank, 2024(3), M1885. https://doi.org/10.3390/M1885






