2-(2,6-Diisopropylphenyl)-1-methylimidazo[1,5-a]quinolin-2-ium Tetrafluoroborate
Abstract
1. Introduction
2. Results and Discussion
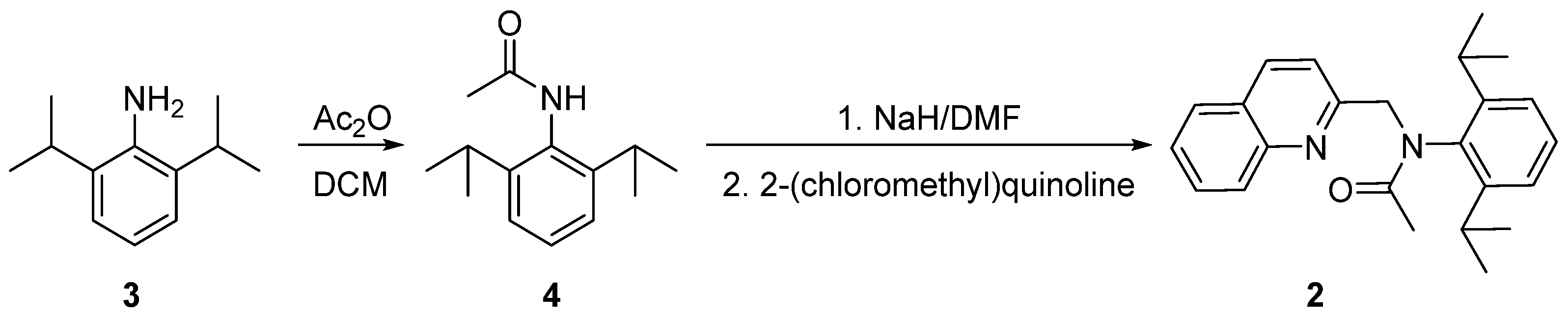
2.1. Synthesis of 2-(2,6-Diisopropylphenyl)-1-methylimidazo[1,5-a]quinolin-2-ium Tetrafluoroborate 1
2.2. NMR Studies
2.3. Optical Properties
3. Materials and Methods
3.1. Synthesis of N-(2,6-Diisopropylphenyl)acetamide 4
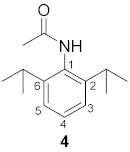
3.2. Synthesis of N-(2,6-Diisopropylphenyl)-N-(quinolin-2-ylmethyl)acetamide 2
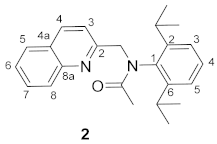
3.3. Synthesis of 2-(2,6-Diisopropylphenyl)-1-methylimidazo[1,5-a]quinolin-2-ium Tetrafluoroborate 1
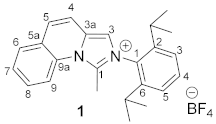
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arduengo, A.J., III; Harlow, R.L.; Kline, M. A stable crystalline carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Flanigan, D.; Romanov-Michailidis, F.; White, N.; Rovis, T. Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.; Köcher, C. N-Heterocyclic Carbenes. Angew. Chem. Int. Ed. Engl. 1997, 36, 2162–2187. [Google Scholar] [CrossRef]
- Kantchev, E.; O’Brien, C.; Organ, M. Palladium Complexes of N-Heterocyclic Carbenes as Catalysts for Cross-Coupling Reactions—A Synthetic Chemist’s Perspective. Angew. Chem. Int. Ed. 2007, 46, 2768–2813. [Google Scholar] [CrossRef]
- Herrmann, W. N-Heterocyclic Carbenes: A New Concept in Organometallic Catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Hahn, F.; Jahnke, M. Heterocyclic Carbenes: Synthesis and Coordination Chemistry. Angew. Chem. Int. Ed. 2008, 47, 3122–3172. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.; Weskamp, T.; Böhm, V. Metal Complexes of Stable Carbenes. Adv. Organomet. Chem. 2001, 48, 1–69. [Google Scholar] [CrossRef]
- O’Brien, C.J.; Kantchev, E.A.B.; Valente, C.; Hadei, N.; Chass, G.A.; Lough, A.; Hopkinson, A.C.; Michael, G.; Organ, M.G. Easily Prepared Air- and Moisture-Stable Pd–NHC (NHC=N-Heterocyclic Carbene) Complexes: A Reliable, User-Friendly, Highly Active Palladium Precatalyst for the Suzuki–Miyaura Reaction. Chem. A Eur. J. 2006, 12, 4743–4748. [Google Scholar] [CrossRef] [PubMed]
- Aldeco-Perez, E.; Rosenthal, A.; Donnadieu, B.; Parameswaran, P.; Frenking, G.; Bertrand, G. Isolation of a C5-Deprotonated Imidazolium, a Crystalline “Abnormal” N-Heterocyclic Carbene. Science 2009, 326, 556–559. [Google Scholar] [CrossRef]
- Arnold, P.L.; Pearson, S. Abnormal N-heterocyclic carbenes. Coord. Chem. Rev. 2007, 251, 596–609. [Google Scholar] [CrossRef]
- Schuster, O.; Yang, L.; Raubenheimer, H.; Albrecht, M. Beyond Conventional N-Heterocyclic Carbenes: Abnormal, Remote, and Other Classes of NHC Ligands with Reduced Heteroatom Stabilization. Chem. Rev. 2009, 109, 3445–3478. [Google Scholar] [CrossRef]
- Vekariya, R. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Ghandi, K. A Review of Ionic Liquids, Their Limits and Applications. GSC 2014, 4, 44–53. [Google Scholar] [CrossRef]
- Benhamou, L.; Chardon, E.; Lavigne, G.; Bellemin-Laponnaz, S.; César, V. Synthetic Routes to N-Heterocyclic Carbene Precursors. Chem. Rev. 2011, 111, 2705–2733. [Google Scholar] [CrossRef]
- Sivaram, H.; Tan, J.; Huynh, H. Syntheses, Characterizations, and a Preliminary Comparative Cytotoxicity Study of Gold(I) and Gold(III) Complexes Bearing Benzimidazole- and Pyrazole-Derived N-Heterocyclic Carbenes. Organometallics 2012, 31, 5875–5883. [Google Scholar] [CrossRef]
- Gillen, J.; Moore, C.; Vuong, M.; Shajahan, J.; Anstey, M.; Alston, J.; Bejger, C. Synthesis and disassembly of an organometallic polymer comprising redox-active Co4S4 clusters and Janus biscarbene linker. ChemComm 2022, 58, 4885–4888. [Google Scholar] [CrossRef]
- Wang, H.; Xia, Y.; Lv, S.; Xu, J.; Sun, Z. Facial and practical synthesis of benzimidazole-based N-heterocyclic carbenes. Tetrahedron Lett. 2013, 54, 2124–2127. [Google Scholar] [CrossRef]
- Tronnier, A.; Pöthig, A.; Metz, S.; Wagenblast, G.; Münster, I.; Strassner, T. Enlarging the π-System of Phosphorescent (C^C*) Cyclometalated Platinum(II) NHC Complexes. Inorg. Chem. 2014, 53, 6346–6356. [Google Scholar] [CrossRef]
- Ullah, F.; Kindermann, M.; Jones, P.; Heinicke, J. Annulated N-Heterocyclic Carbenes: 1,3-Ditolylphenanthreno[9,10-d]imidazol-2-ylidene and Transition Metal Complexes Thereof. Organometallics 2009, 28, 2441–2449. [Google Scholar] [CrossRef]
- Alcarazo, M.; Roseblade, S.; Cowley, A.; Fernández, R.; Brown, J.; Lassaletta, J. Imidazo[1,5-a]pyridine: A Versatile Architecture for Stable N-Heterocyclic Carbenes. J. Am. Chem. Soc. 2005, 127, 3290–3291. [Google Scholar] [CrossRef] [PubMed]
- Lyapchev, R.; Petrov, P.; Dangalov, M.; Vassilev, N. Synthesis and structure elucidation of allyl Pd(II) complexes of NHC ligands derived from substituted imidazo[1,5-a]quinolin-1(2H)-ylidene. J. Organomet. Chem. 2017, 851, 194–209. [Google Scholar] [CrossRef]
- Kriechbaum, M.; Winterleitner, G.; Gerisch, A.; List, M.; Monkowius, U. Synthesis, Characterization and Luminescence of Gold Complexes Bearing an NHC Ligand Based on the Imidazo[1,5-a]quinolinol Scaffold. Eur. J. Inorg. Chem. 2013, 2013, 5567–5575. [Google Scholar] [CrossRef]
- Tao, W.; Nakano, R.; Ito, S.; Nozaki, K. Copolymerization of Ethylene and Polar Monomers by Using Ni/IzQO Catalysts. Angew. Chem. Int. Ed. 2016, 55, 2835–2839. [Google Scholar] [CrossRef] [PubMed]
- Konwar, M.; Hazarika, N.; Sarmah, B.; Das, A. Ruthenium(II)-Catalyzed Oxidative Annulation of Imidazo[1,5-a]quinolin-2-iums Salts and Internal Alkynes via C–H Bond Activation. Chem. Eur. J. 2024, 30, e202401133. [Google Scholar] [CrossRef]
- Konwar, M.; Hazarika, N.; Sarmah, B.; Das, A. Ru/O2-Catalyzed Oxidative C–H Activation/Alkyne Annulation Using Quinoline-Functionalized NHC as a Directing and Functionalizable Group. Org. Lett. 2024, 26, 2965–2970. [Google Scholar] [CrossRef]
- Deligeorgiev, T.; Vasilev, A.; Kaloyanova, S.; Vaquero, J. Styryl dyes—Synthesis and applications during the last 15 years. Color. Technol. 2010, 126, 55–80. [Google Scholar] [CrossRef]
- Said, A.; Kandinska, M.; Vasilev, A.; Grabchev, I. Styryl hemicyanine-DNA assembly for selective Hg2+ sensing and molecular computing. J. Photochem. Photobiol. A 2024, 452, 115590–115600. [Google Scholar] [CrossRef]
- Jeromin, G.; Orth, W.; Rapp, B.; Weiß, W. Seitenkettenchlorierungen von N-Heterocyclen mit Trichlorisocyanursäure (TCC). Chem. Ber. 1987, 120, 649–651. [Google Scholar] [CrossRef]
- Kessler, H. Nachweis innermolekularer beweglichkeit durch nmr-spektrometrie-III: Magnetische nichtäquivalenz geminaler gruppen durch rotationshinderung in achiralen molekülen. Tetrahedron 1968, 24, 1857–1867. [Google Scholar] [CrossRef]
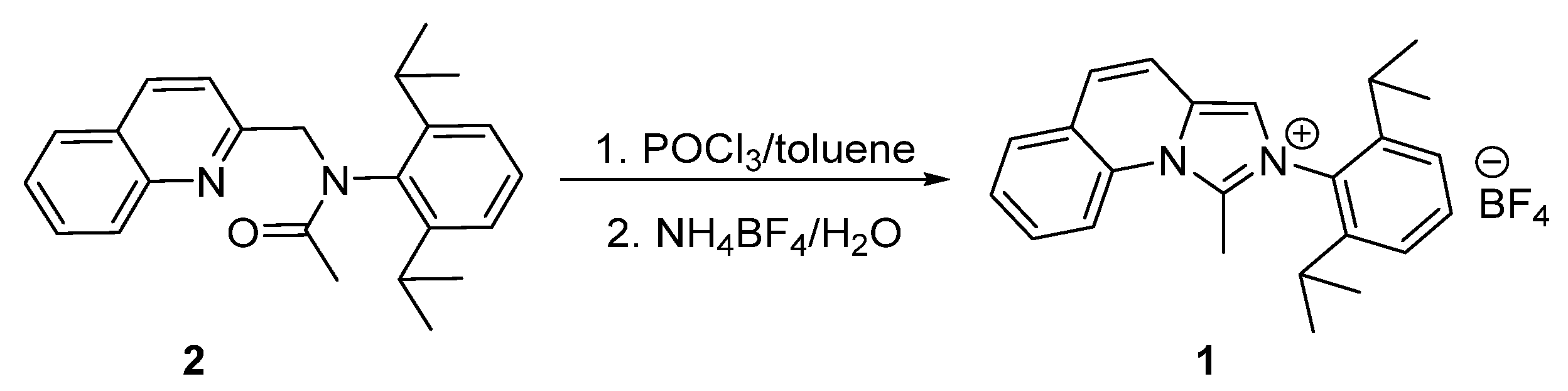

| Solvent | ε, M−1·cm−1 | λabs, nm 1 | λem, nm 2 | Stokes Shift, cm−1 |
|---|---|---|---|---|
| MeOH | 10,443 | 325 | 348; 358 | 2034; 2863 |
| MeCN | 10,106 | 325 | 348; 358 | 2034; 2863 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borislavov, L.; Koleva, I.Z.; Lozanova, V.; Ivanova, M.; Lyapchev, R. 2-(2,6-Diisopropylphenyl)-1-methylimidazo[1,5-a]quinolin-2-ium Tetrafluoroborate. Molbank 2024, 2024, M1868. https://doi.org/10.3390/M1868
Borislavov L, Koleva IZ, Lozanova V, Ivanova M, Lyapchev R. 2-(2,6-Diisopropylphenyl)-1-methylimidazo[1,5-a]quinolin-2-ium Tetrafluoroborate. Molbank. 2024; 2024(3):M1868. https://doi.org/10.3390/M1868
Chicago/Turabian StyleBorislavov, Lyuben, Iskra Z. Koleva, Vesela Lozanova, Maria Ivanova, and Rumen Lyapchev. 2024. "2-(2,6-Diisopropylphenyl)-1-methylimidazo[1,5-a]quinolin-2-ium Tetrafluoroborate" Molbank 2024, no. 3: M1868. https://doi.org/10.3390/M1868
APA StyleBorislavov, L., Koleva, I. Z., Lozanova, V., Ivanova, M., & Lyapchev, R. (2024). 2-(2,6-Diisopropylphenyl)-1-methylimidazo[1,5-a]quinolin-2-ium Tetrafluoroborate. Molbank, 2024(3), M1868. https://doi.org/10.3390/M1868







