5(S)-((3aR,4R,6aR)-2,2-Dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)-2-phenyl-4,5-dihydrooxazole
Abstract
1. Introduction
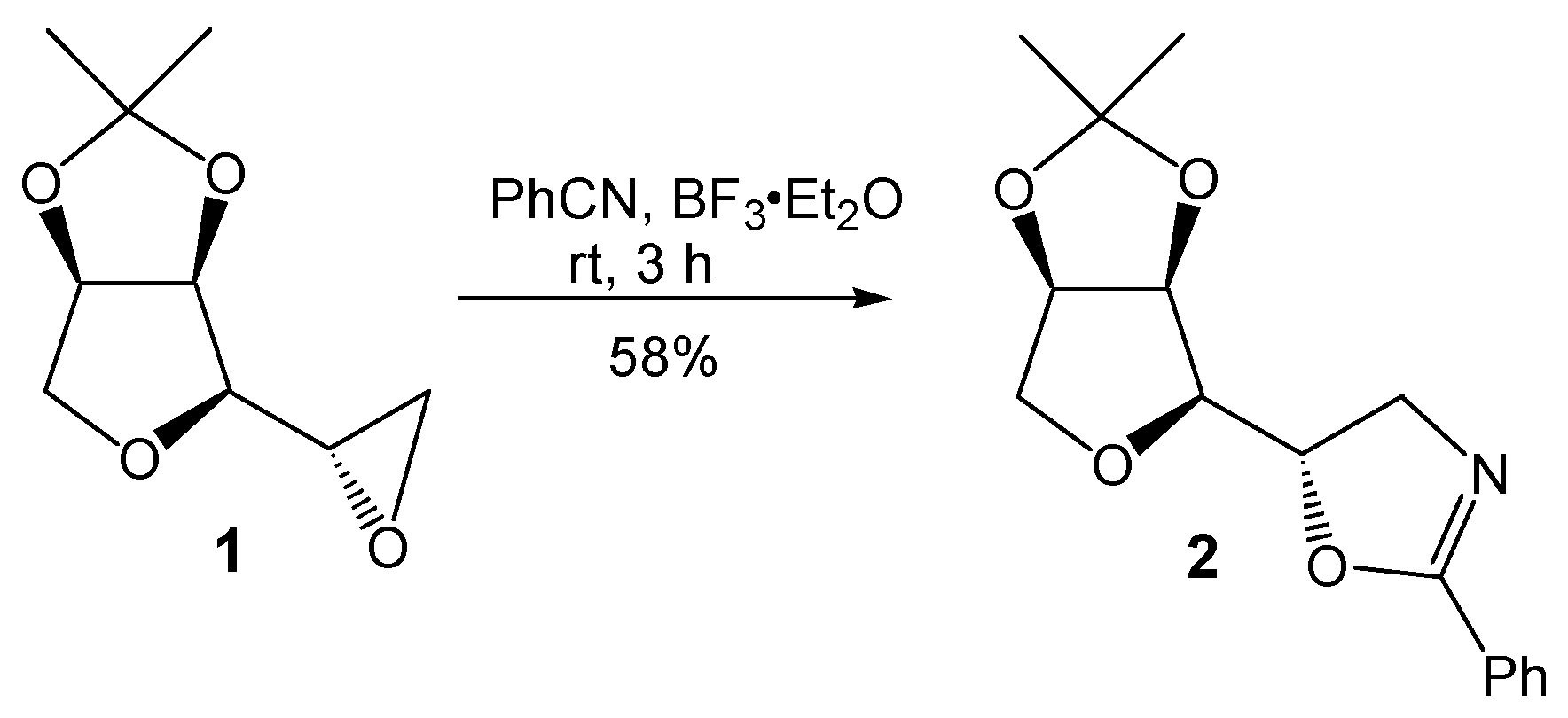
2. Results and Discussion
3. Materials and Methods
5(S)-((3aR,4R,6aR)-2,2-Dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)-2-phenyl-4,5-dihydrooxazole (2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bansal, S.; Halve, A.K. Oxazolines: Their Synthesis and Biological Activity. Int. J. Pharm. Sci. Res. 2014, 5, 4601–4616. [Google Scholar] [CrossRef]
- McManus, H.A.; Guiry, P.J. Recent Developments in the Application of Oxazoline-Containing Ligands in Asymmetric Catalysis. Chem. Rev. 2004, 104, 4151–4202. [Google Scholar] [CrossRef] [PubMed]
- Hargaden, G.C.; Guiry, P.J. Recent Applications of Oxazoline-Containing Ligands in Asymmetric Catalysis. Chem. Rev. 2009, 109, 2505–2550. [Google Scholar] [CrossRef] [PubMed]
- Connon, R.; Roche, B.; Rokade, B.V.; Guiry, P.J. Further Developments and Applications of Oxazoline-Containing Ligands in Asymmetric Catalysis. Chem. Rev. 2021, 121, 6373–6521. [Google Scholar] [CrossRef] [PubMed]
- Saiyed, A.S.; Bedekar, A.V. Amino oxazolines as a new class of organocatalyst for the direct intermolecular asymmetric aldol reaction between acetone and aromatic aldehydes. Tetrahedron Asymmetry 2013, 24, 1035–1041. [Google Scholar] [CrossRef]
- Saegusa, T.; Chujo, Y. Functional polymers based on high hydrophilicity of poly(2-methyl-2-oxazoline). Makromol. Chem. Macromol. Symp. 1990, 33, 31–43. [Google Scholar] [CrossRef]
- Vasilev, K.; Ramiasa-Macgregor, M. Nanoengineered plasma polymer films for biomedical applications. Adv. Mater. Lett. 2018, 9, 42–52. [Google Scholar] [CrossRef]
- Hoogenboom, R. The future of poly(2-oxazoline)s. Eur. Polym. J. 2022, 179, 111521. [Google Scholar] [CrossRef]
- van Es, D.S. Rigid Biobased Building Blocks: Current Developments and Outlook. J. Renew. Mater. 2013, 1, 61–72. [Google Scholar] [CrossRef]
- Ejjiyar, S.; Saluzzo, C.; Massoui, M.; Amouroux, R.; Terry, N.; Coleman, A.W. Synthesis and assembly properties of a series of chiral amphiphilic dihydroxytetrahydrofuran derivatives. J. Phys. Org. Chem. 2001, 14, 1–10. [Google Scholar] [CrossRef]
- Ejjiyar, S.; Saluzzo, C.; Amouroux, R.; Massoui, M. A direct single ring cleavage of isosorbide and isomannide with iodotrimethylsilane. Tetrahedron Lett. 1997, 38, 1575–1576. [Google Scholar] [CrossRef]
- Frump, J.A. Oxazolines. Their Preparation, Reactions, and Applications. Chem. Rev. 1971, 71, 483–504. [Google Scholar] [CrossRef]
- Gant, T.G.; Meyers, A.I. The chemistry of 2-oxazolines (1985–present). Tetrahedron 1994, 50, 2297–2360. [Google Scholar] [CrossRef]
- Smith, R.L.; Norman, R.O.C.; Stillings, M.R. Synthesis of oxazolines from epoxides. J. Chem. Soc. Perkin Trans. I 1975, 1, 1200–1202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boiaryna, L.; Guillarme, S.; Saluzzo, C. 5(S)-((3aR,4R,6aR)-2,2-Dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)-2-phenyl-4,5-dihydrooxazole. Molbank 2024, 2024, M1843. https://doi.org/10.3390/M1843
Boiaryna L, Guillarme S, Saluzzo C. 5(S)-((3aR,4R,6aR)-2,2-Dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)-2-phenyl-4,5-dihydrooxazole. Molbank. 2024; 2024(3):M1843. https://doi.org/10.3390/M1843
Chicago/Turabian StyleBoiaryna, Liliana, Stéphane Guillarme, and Christine Saluzzo. 2024. "5(S)-((3aR,4R,6aR)-2,2-Dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)-2-phenyl-4,5-dihydrooxazole" Molbank 2024, no. 3: M1843. https://doi.org/10.3390/M1843
APA StyleBoiaryna, L., Guillarme, S., & Saluzzo, C. (2024). 5(S)-((3aR,4R,6aR)-2,2-Dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)-2-phenyl-4,5-dihydrooxazole. Molbank, 2024(3), M1843. https://doi.org/10.3390/M1843






