Abstract
Zanthoxylum simulans Hance, commonly known as Sichuan pepper, is a well-known medicinal plant recognized for its potential as a source of bioactive specialized metabolites. As part of our interest in natural antifungal compounds, the present study describes the discovery of an unreported N-alcoxycarbonylbenzo[c]phenanthridinium salt, N-methoxycarbonyl-9,12-dimethoxy-norchelerythrine 1 (a type-III benzo[c]phenanthridine), isolated from Z. simulans seedlings, which were propagated under controlled greenhouse conditions. Six-month seedlings were harvested and subjected to cold acid–base extraction. Chromatographic techniques achieved the isolation of 1 from raw alkaloid extract. The structural elucidation of 1 was accomplished through comprehensive spectroscopic analysis, including nuclear magnetic resonance and high-resolution mass spectrometry. Fusarium oxysporum, a fungal pathogen responsible for substantial agricultural losses, was exposed to different concentrations of the novel compound, exhibiting potent antifungal efficacy (IC50 < 3 µM) and fungicide effects. These findings highlight the potential of benzophenanthridines as antifungal leads and underscore the importance of exploring natural products for agricultural applications.
1. Introduction
Zanthoxylum simulans Hance (Rutaceae), commonly known as the Sichuan pepper, is a deciduous shrub native to Eastern Asia, particularly China [1]. This plant is renowned for its culinary uses, particularly in Sichuan cuisine, for its medicinal properties, and as a multifunctional food [2]. Traditional Chinese medicine has utilized Zanthoxylum species for centuries to treat various ailments, including pain, inflammation, and infections [3]. Several studies have revealed that these therapeutic effects can be attributed to this plant’s rich array of secondary metabolites, particularly phenolics and alkaloids [4].
Alkaloids are a diverse group of naturally occurring compounds that contain basic nitrogen atoms. They are predominantly found in plants and are known for their wide range of pharmacological activities [5]. Benzo[c]phenanthridines, a class of benzylisoquinoline alkaloids [6], are particularly noteworthy for their potent biological activities, including antimicrobial, antiviral, and anticancer properties [7]. The benzophenanthridine structure is characterized by a tetracyclic framework that includes a benzene ring fused to a phenanthridine system and involves three types of benzophenanthridine moieties (I–III) [6]. Sanguinarine (2), chelerythrine (3), and nitidine (4) are well-known type-III benzophenanthridine alkaloids that naturally occur as quaternary ammonium salts (see Figure 1) and have been isolated from various plant sources, including the Papaveraceae and Rutaceae families [7]. The distribution of benzophenanthridine alkaloids across various plant species highlights the importance of exploring different botanical sources for novel compounds with diverse potential applications [6]. The bioactivity of benzophenanthridine alkaloids has acquired significant interest due to their potential as therapeutic agents [8]. Sanguinarine (2), for instance, exhibits potent antimicrobial activity against a range of pathogens, including bacteria, fungi, protozoa, and other relevant biological properties [9]. Chelerythrine (3) has shown capacity as an anticancer agent by inducing apoptosis in cancer cells [10] and even antifungal action [11]. The diverse bioactivities of these alkaloids highlight the potential for developing new drugs and therapeutic agents based on the discovery of bioactive benzophenanthridines [12].
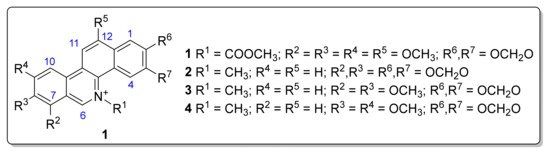
Figure 1.
Structure of type-III benzo[c]phenanthridines 1–4.
Phytopathogens represent a significant threat to global agriculture, leading to substantial economic losses and food security challenges. Fusarium oxysporum, a soil-borne fungal pathogen, is responsible for wilt diseases in a wide range of crops, including tomatoes, bananas, and cucurbits [13,14]. Managing such phytopathogens is critical to ensuring crop health and productivity. However, the increasing resistance of pathogens to conventional fungicides has necessitated the search for alternative strategies [15]. Natural compounds from plants offer a promising path for developing new antifungal agents against phytopathogens [16,17]. Often part of the plant’s defense mechanisms, these compounds can provide effective and sustainable solutions for managing fungal diseases [17]. In recent years, there has been growing interest in the discovery and development of natural antifungal compounds since plant-derived secondary metabolites, such as alkaloids, terpenoids, and phenolics, have shown considerable potential in inhibiting the growth of phytopathogens [18]. These natural products are often less toxic to non-target organisms and more environmentally friendly than synthetic fungicides. Furthermore, exploring plants’ chemical diversity can lead to identifying novel compounds with unique modes of action, thereby overcoming resistance-related issues [19].
Under this context and as part of our interest in antifungal compounds of natural origin, the present study focuses on the discovery and characterization of a novel antifungal type-III benzophenanthridine alkaloid (N-methoxycarbonyl-9,12-dimethoxy-norchelerythrine 1, Figure 1) from Z. simulans seedlings, which were propagated under greenhouse conditions. The seedlings were then harvested and subjected to extraction and purification (through bio-guided fractionation) to isolate the target bioactive compound. Comprehensive spectroscopic techniques, including nuclear magnetic resonance (NMR) and mass spectrometry (MS), were employed for structural elucidation. The antifungal activity of the isolated compound was also evaluated, revealing significant efficacy against Fusarium oxysporum. This study highlights the potential of benzophenanthridines as antifungal leads and underscores the importance of exploring plant-derived compounds for sustainable disease management and combating phytopathogens in agriculture.
2. Results and Discussion
Driven by our interest in the search for antifungal phytochemicals, a screening campaign of exotic plant-derived extracts with antifungal activity was initiated. It was found that the polar alkaloid fraction from seedlings of Z. simulans showed the best activity against F. oxysporum, with 100% inhibition at 50 ppm, using an in vitro assay to evaluate its inhibitory effect on mycelial growth [20]. This finding caught our attention and prompted the study of this alkaloid extract in search of the active components. Consequently, an amorphous brownish solid (compound 1) was obtained after cold acid–base extraction and purification using chromatographic techniques, exhibiting the best antifungal activity (vide infra).
Compound 1 was then characterized and structurally elucidated through spectroscopic techniques. High-resolution mass spectrometry (HRMS) determined its molecular formula to be C24H22NO8+, with a molecular ion peak at m/z 452.1325 [M]+, involving an error of 3.32 ppm. The 1H NMR spectrum of compound 1, recorded in deuterated dimethylsulfoxide (DMSO-d6), exhibited characteristic signals consistent with a polyaromatic alkaloid framework [21]. The 1H NMR spectrum (Figures S1 and S2) showed eleven distinct signals corresponding to singlets (see Table 1). The 13C NMR spectrum (Figures S3 and S4) revealed twenty-four signals. A detailed analysis using HMQC (Figure S5) and APT experiments indicated that these signals corresponded to thirteen quaternary carbons, one methylene carbon, five methine carbons, and five methyl groups.

Table 1.
13C NMR and 1H NMR (DMSO-d6) data of compound 1.
Comparison of the 1H and 13C NMR data (Table 1) with the literature values indicated a similarity to chelerythrine-like quaternary benzo[c]phenanthridiniums salts [22]. However, compound 1 differed from reported type-III benzophenanthridines in the 1H NMR spectrum by four singlets at δH 3.99, 3.88, 3.82, and 3.80, corresponding to the methoxy groups at C-9, C-7, C-12, and C-8, respectively. A singlet at δH 3.68 was attributed to the methoxycarbonyl group at N-5. The aromatic region displayed signals of an ortho-disubstituted benzomethylenedioxy ring system, with two singlets at δH 7.76 and 7.45, corresponding to protons at positions 1 and 4, respectively. Two singlets at δH 7.17 and 6.99 were also observed, assigned to the protons at positions C-11 and C-10. A downfield singlet at δH 9.77 confirmed the presence of a hydrogen H-6 near the quaternary ammonium ion at N-5. The 13C NMR spectrum provided further structural insights into this novel benzophenanthridinium. The spectrum revealed carbon signals for the above-mentioned methoxy groups at δC 56.6, 60.3, 55.9, and 59.8, and the carbonyl carbon at δC 154.8. Signals for aromatic carbons were observed in the range of δC 100 to 157 ppm, with the characteristic signals for the benzomethylenedioxy ring carbons. The methine C-6 appeared at δC 144.9, consistent with the downfield shielding effect of the iminium group. No correlations were observed in the 1H-1H COSY, and the HMQC experiment (Figure S3) demonstrated connectivity between hydrogens and the respective carbons (Table 1).
The HMBC experiment (Figure S6) showed heteronuclear long-range correlations (Figure 2) for finally assigning the structure of 1. Hydrogens of the five methoxy groups correlated with the respective attached carbons at the benzophenanthridine moiety at δC 144.9, 118.1, 152.4, 154.8, and 157.5. The signal at δH 9.77 (H-6) displayed relevant correlations with δC 152.4 and 154.8 (C-7 and carbonyl group, respectively). Indeed, the correlation between H-6 and the carbonyl group of the methoxycarbonyl moiety led to the location of this substituent attached to quaternary nitrogen N-5. Moreover, correlations of H-10 and H-11 allowed the connection of the rest of the type-III benzophenanthridine moiety (Figure 2). In addition, the NOESY experiment (Figure S7) demonstrated spatial correlations that defined the sequential proximity of the system comprising H-4---5-COOCH3---H-6---7-OCH3---8-OCH3---9-OCH3---H-10---H-11---12-OCH3 (Figure 2) and, therefore, supported the previous assignments. Thus, compound 1 was therefore identified as N-methoxycarbonyl-9,12-dimethoxy-norchelerythrine (IUPAC: 1,2,3,6-tetramethoxy-12-(methoxycarbonyl)-[1,3]dioxolo[4′,5′:4,5]benzo[1,2-c]phenanthridin-12-ium) (1), a novel N-alcoxycarbonylbenzo[c]phenanthridinium salt alkaloid from Z. simulans seedlings. Considering the uncommonness of N-alkoxycarbonylpyridinium-containing compounds isolated from natural sources, attempts to determine whether this compound was present in the parent extract by LC-MS were inconclusive due to amount, integrity, or coelution issues. This fact led to the hypothesis that it might be an artifact generated by chloroform-derived phosgene and methanol during the separation procedure. However, bio-guided fractionation successfully oriented the purification of this non-basic alkaloid from the polar alkaloidal extract as the antifungal component. Therefore, future studies will focus on confirming its status as either a natural compound or an artifact.
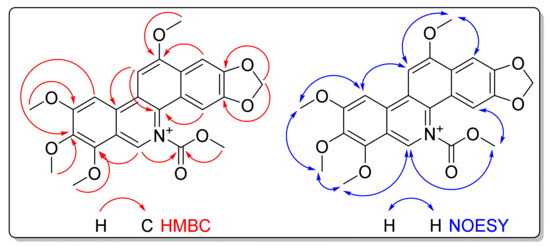
Figure 2.
HMBC and NOESY correlations of compound 1.
The bio-guided fractionation of the polar alkaloidal fraction of Z. simulans seedlings, which exhibited potent antifungal activity against F. oxysporum, led to the isolation of the antifungal compound 1. The isolated compound exhibited a dose-dependent response with an IC50 of 2.46 µM, indicating potent antifungal action through a fungicide effect. Compared to the positive control, iprodione (IC50 = 2.88 µM), compound 1 showed slightly higher activity. Some sanguinarine and chelerythrine derivatives have exhibited antifungal activity against phytopathogenic fungi (IC50 > 14 µg/mL (or >45 µM) [23]; compound 1 exhibited ca. 18-fold better antifungal activity. The observed fungicidal effect can be attributed to the oxidative effect of chelerythrine-like alkaloids on fungal membranes and apoptosis induction of fungal pathogens [11,24].
3. Materials and Methods
3.1. General Experimental Procedures
NMR spectra, including one-dimensional and two-dimensional (1H-1H COSY, HMQC, HMBC, and NOESY) experiments, were recorded on a Bruker Avance 400 spectrometer (400 MHz for 1H and 100 MHz for 13C). Tetramethylsilane (TMS) served as the internal standard, and chemical shifts are reported in δ (ppm) relative to TMS. HRMS data were acquired using an Agilent Technologies 1260 LC system coupled with a quadrupole-time-of-flight (Q-ToF) mass spectrometer and dual Agilent Jet Stream electrospray ionization (AJS ESI) source (Agilent, Santa Clara, CA, USA). Thin-layer chromatography (TLC) was performed on silica gel 60 F254 plates (Merck KGaA, Darmstadt, Germany), utilizing solvent mixtures of n-hexane, chloroform, acetone, and methanol as mobile phases. Developed TLC plates were visualized under UV light at 254 and 365 nm and treated with iodine vapor and Hannessian’s reagent (a mixture of ammonium molybdate, cerium sulfate, and sulfuric acid). Column chromatography (CC), flash chromatography, and vacuum liquid chromatography (VLC) were conducted using silica gel 60 of various particle sizes (Merck KGaA, Darmstadt, Germany).
3.2. Plant Material
The seeds of Z. simulans were purchased from Hooksgreen Herbs Ltd. (Oulton, Staffordshire, UK) and germinated under laboratory conditions. The seeds underwent disinfection using 1% NaClO and 70% ethanol solutions for 1 and 3 min, respectively. Once disinfected, as a pre-germination step, the seeds were previously immersed in a 150-mL mixture of distilled water with eucalyptus tree-derived wood ash (1.0 g) for one week in darkness. Subsequently, they were placed into 150 × 22 mm2 Petri dishes as germination chambers. Thirty seeds were evenly distributed onto absorbent paper and watered thrice weekly with sterile distilled water. The germination chambers were maintained under room conditions (20 °C, natural sunlight, and a 12/12 day/night photoperiod) to facilitate imbibition and subsequent germination. Seeds were considered germinated when the radicle was visibly protruding (at least 1 mm). Afterward, germinated seeds with fully expanded cotyledons were transferred to blond peat (Pindstrup Plus Orange) (Pindstrup Mosebrug A/S, Ryomgaard, Denmark) in 72-well seedbeds, and once the seedlings reached a developmental stage with three true leaves, they were transferred to 2 L bags with a 1:1 sandy loam soil–rice husk mixture to continue propagation under greenhouse conditions. The climate at the test site is classified as subtropical highland with uniform rainfall (Cfb according to the Köppen and Geiger system). It is situated at an altitude of 2560 masl, with an average temperature of 14.1 ± 6.9 °C, 78.2% ± 7.6% relative humidity, and annual precipitation of 1493 mm. Daily irrigation was provided until substrate saturation. Finally, 6-month-old seedlings were then collected for extract preparation.
3.3. Extraction and Isolation
Dried, ground Z. simulans seedlings (143 g) were extracted with 0.5 M HCl (0.5 L), stirring for 24 h at 130 rpm in a temperate orbital shaker at 4 °C. The cold, acidic solution was then filtered and carefully alkalinized to pH 10 using a cold 15% aqueous NH3 solution. Subsequently, the extract was lyophilized, and the solid residue was extracted with methanol to obtain the polar fraction of the alkaloid extract. Finally, the solvent was removed by distillation under reduced pressure to afford the raw polar alkaloid fraction. This fraction (1.3 g) was further fractioned with vacuum liquid chromatography (VLC) by gradient elution using chloroform/MeOH mixtures, resulting in eighteen fractions (Zs1 to Zs18). Each afforded fraction was evaluated for its capacity to inhibit the mycelial growth of F. oxysporum (see the protocol below). Fraction Zs15 was selected since it exhibited the best activity (100% inhibition at 50 ppm), and it was further purified (125 mg) by flash chromatography using chloroform/acetone/methanol (2:2:6) as the mobile phase, producing nine additional fractions (Zs15.1 to f15.9). The most active fraction, Zs15.6 (65 mg), was further depurated by flash chromatography on silica gel with acetone/methanol (4:6), yielding compound 1 (10.5 mg).
N-methoxycarbonyl-9,12-dimethoxy-norchelerythrine (IUPAC: 1,2,3,6-tetramethoxy-12-(methoxycarbonyl)-[1,3]dioxolo[4′,5′:4,5]benzo[1,2-c]phenanthridin-12-ium) (1): brownish amorphous solid (mp 225–227 °C); 1H (400 MHz) and 13C (100 MHz) NMR, see Table 1; HRESIMS (positive mode) m/z 452.1325 [M]+, (calcd. for C24H22NO8+, 452.1340).
3.4. Antifungal Activity
The antifungal efficacy of compound 1 against Fusarium oxysporum was assessed using a 12-well plate amended-medium protocol, as previously described, for evaluating in vitro mycelial growth inhibition [25]. The IC50 value, indicating the concentration required to inhibit 50% of mycelial growth, was determined from a dose–response curve (log(doses) vs. percentage inhibition) using non-linear regression analysis in GraphPad Prism 9.0 (GraphPad, San Diego, CA, USA). Iprodione served as the positive control. After the assay, the highest test concentration was 100 µg/mL. A 2 mm fungal plug was transferred from the highest-dose well to a fresh medium-containing well to differentiate between fungistatic (inhibiting fungal growth) and fungicidal (fungus-killing) effects. The subsequent growth or lack thereof indicated whether the effect of compound 1 was fungistatic or fungicidal.
4. Concluding Remarks
A novel N-alcoxycarbonylbenzo[c]phenanthridinium salt (a type-III benzo[c]phenanthridine) was isolated from the polar alkaloid fraction of Z. simulans seedlings. Based on the comprehensive analysis of NMR and HRMS spectral data, the structure of the novel compound isolated from Z. simulans seedlings was determined to be 1,2,3,6-tetramethoxy-12-(methoxycarbonyl)-[1,3]dioxolo[4′,5′:4,5]benzo[1,2-c]phenanthridin-12-ium (1). The test fraction was obtained from Z. simulans seedlings, which were propagated under controlled greenhouse conditions. Compound 1 exhibited noteworthy antifungal activity against F. oxysporum (IC50 = 2.44 µM), implying a fungicide effect. The discovery of 1 highlights promising avenues for research into type-III benzophenanthridine alkaloids and their antifungal applications against phytopathogens.
Supplementary Materials
Figure S1. 1H NMR spectrum of 1 (400 MHz, DMSO-d6), Figure S2. Expansion of 1H RMN spectrum of 1 (3.5–10.0 ppm), Figure S3. 13C NMR spectrum of 1 (100 MHz, DMSO-d6), Figure S4. Expansion of 13C NMR spectrum of 1 (50–160 ppm), Figure S5. HMQC experiment of 1, Figure S6. HMBC experiment of 1, Figure S7. NOESY experiment of 1, Figure S8. HRMS spectrum of 1.
Author Contributions
Conceptualization, E.C.-B.; methodology and investigation, D.C.-L. and E.C.-B.; formal analysis, D.C.-L., D.Q. and E.C.-B.; resources, D.Q. and E.C.-B.; writing—original draft preparation, D.C.-L. and E.C.-B.; writing—review and editing, D.C.-L. and D.Q.; supervision, project administration, and funding acquisition, E.C.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Vicerrectoría de Investigaciones at Universidad Militar Nueva Granada (UMNG) through the project EXT-CIAS-3854, validity 2023.
Data Availability Statement
Data are available from the authors upon reasonable request.
Acknowledgments
The authors thank UMNG for the financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lim, T.K. (Ed.) Zanthoxylum Simulans. In Edible Medicinal and Non-Medicinal Plants: Volume 4, Fruits; Springer: Dordrecht, The Netherlands, 2012; pp. 904–911. ISBN 978-94-007-4053-2. [Google Scholar]
- Ivane, N.M.A.; Haruna, S.A.; Zekrumah, M.; Roméo Elysé, F.K.; Hassan, M.O.; Hashim, S.B.H.; Tahir, H.E.; Zhang, D. Composition, Mechanisms of Tingling Paresthesia, and Health Benefits of Sichuan Pepper: A Review of Recent Progress. Trends Food Sci. Technol. 2022, 126, 1–12. [Google Scholar] [CrossRef]
- Nooreen, Z.; Tandon, S.; Yadav, N.P.; Kumar, P.; Xuan, T.D.; Ahmad, A. Zanthoxylum: A Review of Its Traditional Uses, Naturally Occurring Constituents and Pharmacological Properties. Curr. Org. Chem. 2019, 23, 1307–1341. [Google Scholar] [CrossRef]
- Okagu, I.U.; Ndefo, J.C.; Aham, E.C.; Udenigwe, C.C. Zanthoxylum Species: A Review of Traditional Uses, Phytochemistry and Pharmacology in Relation to Cancer, Infectious Diseases and Sickle Cell Anemia. Front. Pharmacol. 2021, 12, 713090. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Laines-Hidalgo, J.I.; Muñoz-Sánchez, J.A.; Loza-Müller, L.; Vázquez-Flota, F. An Update of the Sanguinarine and Benzophenanthridine Alkaloids’ Biosynthesis and Their Applications. Molecules 2022, 27, 1378. [Google Scholar] [CrossRef]
- Han, N.; Yang, Z.; Liu, Z.; Liu, H.; Yin, J. Research Progress on Natural Benzophenanthridine Alkaloids and Their Pharmacological Functions: A Review. Nat. Prod. Commun. 2016, 11, 1934578X1601100838. [Google Scholar] [CrossRef]
- Peng, R.; Xu, M.; Xie, B.; Min, Q.; Hui, S.; Du, Z.; Liu, Y.; Yu, W.; Wang, S.; Chen, X.; et al. Insights on Antitumor Activity and Mechanism of Natural Benzophenanthridine Alkaloids. Molecules 2023, 28, 6588. [Google Scholar] [CrossRef]
- Huang, L.-J.; Lan, J.-X.; Wang, J.-H.; Huang, H.; Lu, K.; Zhou, Z.-N.; Xin, S.-Y.; Zhang, Z.-Y.; Wang, J.-Y.; Dai, P.; et al. Bioactivity and Mechanism of Action of Sanguinarine and Its Derivatives in the Past 10 Years. Biomed. Pharmacother. 2024, 173, 116406. [Google Scholar] [CrossRef]
- Kang, K.; Jiang, H.; Zhang, S.; Cheng, B. Antitumor Effects of Chelerythrine: A Literature Review. Nat. Prod. Commun. 2022, 17, 1934578X221103028. [Google Scholar] [CrossRef]
- Wei, Q.; Cui, D.; Liu, X.; Chai, Y.; Zhao, N.; Wang, J.; Zhao, M. In Vitro Antifungal Activity and Possible Mechanisms of Action of Chelerythrine. Pestic. Biochem. Physiol. 2020, 164, 140–148. [Google Scholar] [CrossRef]
- Basu, A.; Kumar, G.S. Interaction of the Putative Anticancer Alkaloid Chelerythrine with Nucleic Acids: Biophysical Perspectives. Biophys. Rev. 2020, 12, 1369–1386. [Google Scholar] [CrossRef]
- Bhar, A.; Jain, A.; Das, S. Soil Pathogen, Fusarium Oxysporum Induced Wilt Disease in Chickpea: A Review on Its Dynamicity and Possible Control Strategies. Proc. Indian Natl. Sci. Acad. 2021, 87, 260–274. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Ahmad, K.; Bashir Kutawa, A.; Siddiqui, Y.; Saad, N.; Geok Hun, T.; Hata, E.M.; Hossain, M.I. Biology, Diversity, Detection and Management of Fusarium Oxysporum f. Sp. Niveum Causing Vascular Wilt Disease of Watermelon (Citrullus Lanatus): A Review. Agronomy 2021, 11, 1310. [Google Scholar] [CrossRef]
- Haruna, S.G.; Yahuza, L.; Tijjani, I. Management of Fusarium Wilt of Tomato (Fusarium Oxysporum f. Sp. Lycopersici) and Related Soil-Borne Diseases Using Eco-Friendly Methods: A Review. Asian J. Res. Crop Sci. 2024, 9, 154–168. [Google Scholar] [CrossRef]
- Deresa, E.M.; Diriba, T.F. Phytochemicals as Alternative Fungicides for Controlling Plant Diseases: A Comprehensive Review of Their Efficacy, Commercial Representatives, Advantages, Challenges for Adoption, and Possible Solutions. Heliyon 2023, 9, e13810. [Google Scholar] [CrossRef]
- Santra, H.K.; Banerjee, D. Natural Products as Fungicide and Their Role in Crop Protection. In Natural Bioactive Products in Sustainable Agriculture; Singh, J., Yadav, A.N., Eds.; Springer: Singapore, 2020; pp. 131–219. ISBN 978-981-15-3024-1. [Google Scholar]
- Hussain, T.; Singh, S.; Danish, M.; Pervez, R.; Hussain, K.; Husain, R. Natural Metabolites: An Eco-Friendly Approach to Manage Plant Diseases and for Better Agriculture Farming. In Natural Bioactive Products in Sustainable Agriculture; Singh, J., Yadav, A.N., Eds.; Springer: Singapore, 2020; pp. 1–13. ISBN 978-981-15-3024-1. [Google Scholar]
- Thind, T.S. Changing Trends in Discovery of New Fungicides: A Perspective. Indian Phytopathol. 2021, 74, 875–883. [Google Scholar] [CrossRef]
- Marentes-Culma, R.; Orduz-Díaz, L.L.; Coy-Barrera, E. Targeted Metabolite Profiling-Based Identification of Antifungal 5-n-Alkylresorcinols Occurring in Different Cereals against Fusarium Oxysporum. Molecules 2019, 24, 770. [Google Scholar] [CrossRef]
- Sečkářová, P.; Marek, R.; Dostál, J.; Dommisse, R.; Esmans, E.L. Structural Studies of Benzophenanthridine Alkaloid Free Bases by NMR Spectroscopy. Magn. Reson. Chem. 2002, 40, 147–152. [Google Scholar] [CrossRef]
- Marek, R.; Toušek, J.; Dostál, J.; Slavík, J.; Dommisse, R.; Sklenář, V. 1H and 13C NMR Study of Quaternary Benzo[c]Phenanthridine Alkaloids1. Magn. Reson. Chem. 1999, 37, 781–787. [Google Scholar] [CrossRef]
- Yang, X.-J.; Miao, F.; Yao, Y.; Cao, F.-J.; Yang, R.; Ma, Y.-N.; Qin, B.-F.; Zhou, L. In Vitro Antifungal Activity of Sanguinarine and Chelerythrine Derivatives against Phytopathogenic Fungi. Molecules 2012, 17, 13026–13035. [Google Scholar] [CrossRef]
- Gong, Y.; Li, S.; Wang, W.; Li, Y.; Ma, W.; Sun, S. In Vitro and in Vivo Activity of Chelerythrine against Candida Albicans and Underlying Mechanisms. Future Microbiol. 2019, 14, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Cely-Veloza, W.; Quiroga, D.; Coy-Barrera, E. Quinolizidine-Based Variations and Antifungal Activity of Eight Lupinus Species Grown under Greenhouse Conditions. Molecules 2022, 27, 305. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).