Abstract
An unsymmetrical PCN palladium pincer complex 2-{(E)-{{(R)-1-[(1R,5S,6R)-3,3-dimethyl-2,4,7-trioxabicyclo[3.3.0]octan-6-yl]ethyl}imino}methyl}-6-{[(diphenylphosphino)oxy] phenyl}palladium(II) chloride based on an iminophosphinite ligand bearing two fused five-membered cycles, one of which containing a THF ring, was prepared in an eight-reaction sequence from a sustainable and enantiopure starting material, isosorbide, in a 20% overall yield.
1. Introduction
Since the first Shaw [1] and van Kolten [2] reports on the symmetric PCP and NCN pincer complex of metal transition, these type of complexes have attracted increasing attention due to their particular properties [3] and their use in wide-ranging reactions such as Kharasch and aldol Michael additions, Heck and Suzuki couplings, dehydrogenation, hydrogen transfer, etc. [4,5,6,7,8,9,10,11,12,13,14,15].
For C-C coupling reactions, as palladium complexes are considered to be the catalysts of choice, a large number of symmetrical [4,5,7,10,11], unsymmetrical [6,8], and chiral [11,13] palladium pincer complexes have been investigated. From all of them, only a few unsymmetric PCN pincer palladium (II) complexes with iminophosphinite ligands have been described thus far. Some of them have been synthesized by Xia for Suzuki or Kumada type couplings [16,17,18]. As isohexide derivatives have shown their efficiency as ligands in organometallic catalysis or as organocatalysts [19,20], we report herein the synthesis of an optical active non-symmetrical iminophosphinite PCN pincer palladium (II) complex derived from isosorbide, which is a sustainable material.
2. Results and Discussion
Starting from commercially available isosorbide, the synthesis of the targeted chiral PCN pincer palladium(II) Complex 6 was carried out in eight steps. The first three steps involved the preparation of the bicyclic Alcohol 1 according to our previous reports [21,22,23]. Next, mesylation of (S)-alcohol 1 yielded (S)-mesylate 2, which, after treatment with an excess sodium azide, afforded the corresponding (R)-azide 3 as a single stereoisomer. Slight hydrogenation of the latter led to the desired (R)-amine 4 (Scheme 1).
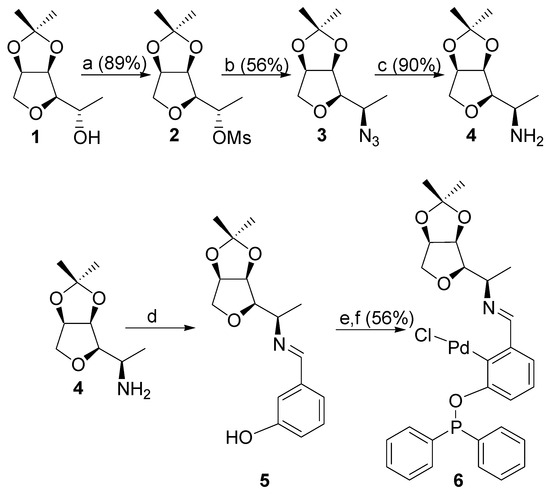
Scheme 1.
(a) MsCl, Et3N, CH2Cl2, 15 h. (b) NaN3, DMF/H2O, 140 °C, 48 h. (c) H2, 10% Pd/C, EtOH, r.t. 15 h. (d) 3-hydroxybenzaldehyde, CH2Cl2, MgSO4, 15 h. (e) Ph2PCl, Et3N, toluene, 110 °C, 1 h. (f) PdCl2, 110 °C, 15 h.
The targeted NPC palladium(II) Complex 6 (Scheme 1) was obtained in a one-pot (d, e, f, 56% global yield) strategy. The condensation of Amine 4 with 3-hydroxybenzaldehyde led to the Schiff-base 5 (Step d), which was involved in a phosphorylation (Step e) and a final metalation (Step f). Complex 6 was isolated as a yellow powder after purification over silica gel.
3. Materials and Methods
3.1. General
Commercially available reagents were used as received. Dichloromethane and tetrahydrofuran were dried with an activated alumina column (Glass Technology GTS 100). Dry triethylamine and toluene were distillated on CaH2. Thin Layer Chromatography (TLC) was performed on precoated aluminum (200 μm) Merck silica gel (DC Kieselgel 60 F254), and it was visualized using UV irradiation or via staining with a ready-to-use Aldrich phosphomolybdic acid (PMA) solution reagent. Column chromatography was carried out with Merck silica gel (Kiesegel 60, 0.063–0.200 mm). Optical rotations [α]D25 were carried out on a JASCO P-2000 Polarimeter. Melting points were carried out on a Barnstead Electrothermal IA9300 instrument and were not corrected. The NMR spectra were acquired on a Bruker AVANCE 400 spectroscope operating at 400, 162, and 100 MHz for 1H, 31P, and 13C nuclei, respectively. All chemical shifts (δ values) are given in parts per million (ppm) and all homo-heterocoupling patterns (nJH,H, nJH,P and nJC,P values) are given in hertz (Hz). Multiplicity is indicated as singlet (s), broad singlet (bs), doublet (d), triplet (t), quadruplet (q), and multiplet (m). The 1H and 13C NMR spectra were referenced against TMS and 31P NMR against triphenylphosphate as the external reference. ATR-FTIR spectra were recorded with an Agilent Technologies apparatus. Only the relevant absorption maxima, νmax (cm−1), are listed throughout as “s” (strong), “m” (medium), or “w” (weak). High resolution mass spectroscopy (HRMS) was carried out with a Waters Micromass GCT Premier Device apparatus.
3.2. Synthesis and Characterization
Alcohol 1 was synthetized and exhibited analytical data in full agreement with the Ejjiyar procedure [21,22]. For the NMR assignment and for a better understanding, adapted numbering based on IUPAC nomenclature was chosen. Copies of NMR spectra (S1–S11) and HRMS of the complex 6 (S12) are available in the Supplementary Material.
3.2.1. (S)-1-{[(1R,5S,6R)-3,3-Dimethyl-2,4,7-trioxabicyclo[3.3.0]octan-6-yl]ethyl}methanesulfonate (2)
At 0 °C, under argon and stirring, methanesulfonyl chloride (1.12 g, 0.76 mL, 9.82 mmol, 1.05 equiv) and triethylamine (0.90 mg, 1.40 mL, 10.00 mmol, 1.10 equiv) were successively added to a solution of Alcohol 1 (1.75 g, 9.31 mmol) in anhydrous dichloromethane (40 mL). After stirring overnight, TLC monitoring (cyclohexane/AcOEt, 6:4 v/v) indicated completion of the reaction. The reaction mixture was washed with water (20 mL), with HCl aq (1N, 10 mL), and then with water (20 mL) and brine (20 mL). Finally, the organic phase was dried over MgSO4, which was filtered off and concentrated in a vacuum to afford Mesylate 2 (2.22 g, 8.29 mmol, 89% yield) as a pure analytical sample. Compound 2 was used without further purification.
White solid m.p. 128 °C (DCM). Rf = 0.41 (cyclohexane/AcOEt, 6:4 v/v, PMA). [α]D25 = −15.6 (c 0.5, CH2Cl2). 1H NMR (400 MHz, CDCl3) 1.30 (3H, s, Mea); 1.47 (3H, s, Meb); 1.51 (3H, d, 3JH2H1 = 6.5 Hz, H-2); 3.06 (3H, s, H-1″); 3.48 (1H, dd, 3JH6′H2 = 6.6 Hz, 3JH6′H5′ = 3.6 Hz, H-6′); 3.50 (1H, dd, 2JH8′aH8′b = 10.8 Hz, 3J H8′a H1′ = 3.7 Hz, H-8′a); 4.04 (1H, d, 2JH8′bH8′a = 10.8 Hz, H-8′b), 4.63 (1H, dd, 3JH5′H1′ = 6.1 Hz, 3JH5′6′ = 3.6 Hz, H-5′); 4.80 (1H, dd, 3JH1′H5′ = 6.1 Hz, 3JH1′H8′a = 3.7 Hz, H-1′); 4.90 (1H, dq, 3JH1H6′ = 6.6 Hz, 3JH1H2 = 6.5 Hz, H-1). 13C NMR (100 MHz, CDCl3) 18.0 (Mea); 24.0 (Meb); 26.0 (C-2); 38.3 (C-1”); 73.0 (C-8′); 79.7 (C-1); 80.2 (C-5′); 81.1 (C-1′); 84.1 (C-6′); and 112.4 (C-3′). HRMS (FDI) m/z [M-CH3]+ 251.0609; Calcd. for C9H15O6S 251.0589).
3.2.2. (R)-1-{[(1R,5S,6R)-3,3-Dimethyl-2,4,7-trioxabicyclo[3.3.0]octan-6-yl]ethyl}azide (3)
A solution of Mesylate 2 (1.72 g, 6.42 mmol) and sodium azide (2.1 g, 32.1 mmol, 5 equiv) in a DMF/water mixture (5:2 v/v, 20 mL) was heated at 140 °C for 48 h. The mixture was cooled to room temperature before adding water. The solution was extracted four times with diethyl ether. The combined organic phases were dried over MgSO4 and concentrated in a vacuum. The crude azide was purified by column chromatography (cyclohexane/AcOEt, 95:5 then 60:40 v/v) to give Azide 3 (0.75 g, 3.52 mmol, 56%) as a colorless oil.
Rf = 0.5 (cyclohexane/AcOEt, 8:2 v/v, PMA); [α]D25 = −118 (c 1.0, CHCl3). 1H NMR (400 MHz, CDCl3) 1.35 (3H, s, Mea); 1.40 (3H, d, 3JH2H = 6.6 Hz, H-2); 1.48 (3H, s, H, Meb); 3.17 (1H, dd, 3JH6′H1 = 9.2 Hz, 3JH6′H5′ = 3.5 Hz, H-6′); 3.48 (1H, dd, 2JH8′aH8′b = 10.8 Hz, 3JH8′aH1′ = 3.6 Hz, H-8′a); 3.84 (1H, dq, 3JH1H6′ = 9.2 Hz, 3JH1H2 = 6.6 Hz, H-1); 4.01 (1H, d, 2JH8′bH8′a = 10.8 Hz, H-8′b); 4.71 (1H, dd, 3JH5′H1′ = 6.1 Hz, 3JH5′H6′ = 3.5 Hz, H-5′); 4.77 (1H, dd, 3JH1′H5′ = 6.1 Hz, 3JH1′H8′a = 3.6 Hz, H-1′). 13C NMR (100 MHz, CDCl3) 17.1 (C-2); 24.8 (Mea); 26.0 (Meb); 55.3 (C-1); 73.0 (C-8′); 80.2 (C-5′); 80.9 (C-1′); 84.9 (C-6′); and 112.3 (C-3′). IR (neat, νmax) 2983 (s), 2937 (s), 2853 (s), 2089 (s, N3), 1458 (w), 1376 (m), 1264 (s), 1165 (w), 1112 (s), and 1073 (m) cm−1. HRMS (ESI) m/z [M+Na]+ 236.1003. (Calcd. for C9H15O6N3Na 236.1006.)
3.2.3. (R)-1-[(1R,5S,6R)-3,3-Dimethyl-2,4,7-trioxabicyclo[3.3.0]octan-6-yl]ethylamine (4)
A solution of Azide 3 (666 mg; 3.13 mmol) and Pd/C 10% (71 mg) in ethanol (18 mL) was stirred at room temperature under hydrogen (4 bar) overnight. The mixture was filtered over Celite, and the filtrate was concentrated in a vacuum to give Amine 4 (542 mg, 2.90 mmol, 88%) as a slightly yellow oil, which was used without further purification.
[α]D20 = 70.1 (c 1.0, CHCl3).1H NMR (400 MHz, CDCl3) 1.23 (3H, d, 3JH2H1 = 6.5 Hz, H-2); 1.33 (3H, s, Mea); 1.49 (3H, s, Meb); 1.75 (2H, bs, NH2); 3.09 (1H, dd, 3JH6′H1 = 8.1 Hz, 3JH6′H5′ = 3.0 Hz, H-6′); 3.22-3.35 (1H, m, H-1); 3.48 (1H, dd, 2JH8′aH8′b = 10.8 Hz, 3JH8′H1′ = 3.4 Hz, H-8′a); 4.02 (1H, d, 2JH8′bH8′a = 10.8 Hz, H-8′b); 4.73 (1H, dd, 3JH5′H1′ = 6.2 Hz, 3JH5′H6′ = 3.0 Hz, H-5′); 4.76 (1H, dd, 3JH1′H5′ = 6.2 Hz, and 3JH1′H8′a = 3.4 Hz, H-1′). 13C NMR (100 MHz, CDCl3) 21.0 (C-2); 24.7 (Mea); 26.0 (Meb); 46.2 (C-1); 72.4 (C-8′); 80.6 (C-5′); 81.0 (C-1′); 88.0 (C-6′); and 112.0 (C-3′). IR (neat,νmax) 3376 (m, NH2), 2982 (s), 2937 (s), 2851 (s), 1460 (w), 1374 (m), 1270 (s), 1168 (w), 1100 (s), and 1091 (m) cm−1. HRMS (ESI) m/z [M+H]+ 188.1279. (Calcd. for C9H18NO3 188.1281.)
3.2.4. 2-{(E)-{{(R)-1-[(1R,5S,6R)-3,3-Dimethyl-2,4,7-trioxabicyclo[3.3.0]octan-6-yl]octan-6-yl]ethyl}imino}methyl}-6-{[(diphenylphosphino)oxy]phenyl}palladium(II) chloride (6)
Under argon, a solution of Amine 4 (102.8 mg, 0.551 mmol) and 3- hydroxybenzaldehyde (67.3 mg, 0.551 mmol) in dry dichloromethane (3 mL) in the presence of MgSO4 was stirred overnight at room temperature. The mixture was filtered, and the solvent evaporated in a vacuum to give a crude Imine 5, which was immediately dissolved in dry toluene (5 mL) under argon. Chlorodiphenylphosphine (145 µg, 122 µL, 0.660 mmol, 1.2 equiv) and triethylamine (67 µg, 92 µL, 0.660 mmol, 1.2 equiv) were then added, and the solution was heated under reflux for 1 h. The solution was allowed to cool to room temperature and palladium (II) chloride (97.5 mg, 0.550 mmol) was added. The mixture was heated to reflux overnight, cooled to room temperature, and then filtered. The filtrate was concentrated under vacuum and the crude product purified by column chromatography (CH2Cl2 then CH2Cl2/Et2O, 97:3 v/v) to give Complex 6 (190.2 mg, 0.309 mmol, 56%) as a yellow powder.
Rf = 0.1 (CH2Cl2, UV); mp > 250 °C; 1H NMR (400 MHz, CDCl3) 1.24 (3H, s, H-8 or H-9); 1.49 (3H, s, H-9 or H-8); 1.70 (3H, d, 3JH2H1 = 6.5 Hz, H-2); 3.57 (1H, dd, 2JH8′aH8′b = 10.7 Hz, 3JH8′aH1′ = 3.6 Hz, H-8′a); 3.98 (1H, d, 2JH8′bH8′a = 10.7 Hz, H-8′b); 4.11 (1H, dq, 3JH1H6′ = 8.5 Hz, 3JH1H2 = 6.5 Hz, H-1); 4.43 (1H, dd, 3J H6′H1 = 8.5 Hz, 3JH6′H5′ = 3.5 Hz, H-6′); 4.70 (dd, 1H, 3J H5′H1′ = 6.2 Hz, 3J H5′H6′ = 3.5 Hz, H-5′); 4.75 (1H, dd, 3JH1′H5′ = 6.2 Hz, 3JH1′H8′a = 3.6 Hz, H-1′); 6.93-6.96 (1H, m, HAr); 7.09-7.44 (1H, m, HAr); 7.46- 7.58 (6H, m, HAr); 7.97-8.05 (4H, m, HAr); and 8.21 (1H, d, J = 4.9 Hz, H10). 31P NMR (162 MHz, CDCl3) 152.24. 13C NMR (100 MHz, CDCl3) 18.7 (C-1); 24.9 (C-8); 26.1 (C-9); 66.6 (C-2); 72.7 (C-6); 80.3 (C-5); 81.5 (C-4); 82.5 (C-3); 111.8 (C-7); 114.8 (d, JCP = 17.6 Hz); 122.6, 128.8 (d, JCP = 12.0 Hz), 131.6, 131.8, 132.2, 132.3, 132.7 (d, JCP = 55.5 Hz); 153.7; 145.7; 162.1 (d, JCP = 9.6 Hz); and 175.1 (d, JCP = 3.2 Hz) (18CAr, C-10). HRMS (FD+) m/z (100%) [M-Cl]+ 579.1213. (Calcd. for C28H28NO4PPd 580.0869.) m/z (42%) [M]+ 615.0492. (Calcd. for C28H28NO4ClPPd 615.0557.)
4. Conclusions
We synthesized and characterized iminophosphinite palladium (II) complexes from isosorbide, which is a sustainable material, in an eight-step sequence with an overall yield of 20%.
Supplementary Materials
Figure S1: 1H NMR spectrum of Compound 2 in CDCl3. Figure S2: 13C NMR spectrum of Compound 2 in CDCl3. Figure S3: HSQC spectrum of Compound 2 in CDCl3. Figure S4: 1H NMR spectrum of Compound 3 in CDCl3. Figure S5: 13C NMR spectrum of Compound 3 in CDCl3. Figure S6: HSQC spectrum of Compound 3 in CDCl3. Figure S7: 1H NMR spectrum of Compound 4 in CDCl3. Figure S8: 13C NMR spectrum of Compound 4 in CDCl3. Figure S9: 1H NMR spectrum of Complex 6 in CDCl3. Figure S10: 31P {1H} NMR spectrum of Complex 6 in CDCl3. Figure S11: 13C NMR spectrum of Complex 6 in CDCl3. Figure S12: HRMS spectrum of Complex 6 in CDCl3.
Author Contributions
Conceptualization, S.G.; validation, S.G. and C.S.; investigation, S.G.; writing—original draft preparation, C.S.; writing—review and editing, S.G. and C.S.; visualization, C.S.; supervision, C.S.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors would like to thank the Ministère de la Recherche and the CNRS for their financial support. They also wish to extend thanks to Alexandre Besnard and Arnaud Martel for their contribution to the HRMS analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Moulton, C.J.; Shaw, B.L. Transition metal-carbon bonds. Part XLII. Complexes of nickel, palladium, platinum, rhodium and iridium with the tridentate ligand 2,6-bis[(di-t-butylphosphino)methyl]phenyl. J. Chem. Soc. Dalton Trans. 1976, 1020–1024. [Google Scholar] [CrossRef]
- Van Koten, G.; Timmer, K.; Noltes, J.G.; Spek, A.L. A novel type of Pt–C interaction and a model for the final stage in reductive elimination processes involving C–C coupling at Pt; synthesis and molecular geometry of [1,N,N′-η-2,6-bis{(dimethylamino)methyl}-toluene]iodoplatinum(II) tetrafluoroborate. J. Chem. Soc. Chem. Commun. 1978, 250–252. [Google Scholar] [CrossRef]
- Rietveld, M.H.P.; Grove, D.M.; van Koten, G. Recent Advances in the Organometallic Chemistry of Aryldiamine Anions That Can Function as N,C,N′- and C,N,N′-Chelating Terdentate “Pincer” Ligands: An Overview. New J. Chem. 1997, 21, 751–771. [Google Scholar] [CrossRef]
- Albrecht, M.; van Koten, G. Platinum Group Organometallics Based on “Pincer” Complexes: Sensors, Switches, and Catalysts. Angew. Chem. Int. Ed. 2001, 40, 3750–3781. [Google Scholar] [CrossRef]
- Singleton, J.T. The uses of pincer complexes in organic synthesis. Tetrahedron 2003, 59, 1837–1857. [Google Scholar] [CrossRef]
- van der Boom, M.E.; Milstein, D. Cyclometalated Phosphine-Based Pincer Complexes: Mechanistic Insight in Catalysis, Coordination, and Bond Activation. Chem. Rev. 2003, 103, 1759–1792. [Google Scholar] [CrossRef] [PubMed]
- Peris, E.; Crabtree, R.H. Recent homogeneous catalytic applications of chelate and pincer N-heterocyclic carbenes. Coord. Chem. Rev. 2004, 248, 2239–2246. [Google Scholar] [CrossRef]
- Dupont, J.; Consorti, C.S.; Spencer, J. The Potential of Palladacycles: More Than Just Precatalysts. Chem. Rev. 2005, 105, 2527–2572. [Google Scholar] [CrossRef] [PubMed]
- Morales-Morales, D.; Jensen, C.M. (Eds.) The Chemistry of Pincer Compounds; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Serrano-Becerra, J.M.; Morales-Morales, D. Applications in Catalysis and Organic Transformations Mediated by Platinium Group PCP and PNP Aromatic-Based Pincer Complexes: Recent Advances. Curr. Org. Synth. 2009, 6, 169–192. [Google Scholar] [CrossRef]
- Zhang, B.-S.; Wang, W.; Shao, D.-D.; Hao, X.-Q.; Gong, J.-F.; Song, M.-P. Unsymmetrical Chiral PCN Pincer Palladium(II) and Nickel(II) Complexes of (Imidazolinyl)aryl Phosphinite Ligands: Synthesis via Ligand C−H Activation, Crystal Structures, and Catalytic Studies. Organometallics 2010, 29, 2579–2587. [Google Scholar] [CrossRef]
- Selander, N.; Szabó, K.J. Catalysis by Palladium Pincer Complexes. Chem. Rev. 2011, 111, 2048–2076. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.-L.; Hao, X.-Q.; Gong, J.-F.; Song, M.-P. Symmetrical and unsymmetrical pincer complexes with group 10 metals: Synthesis via aryl C-H activation and some catalytic applications. Dalton Trans. 2011, 40, 5135–5150. [Google Scholar] [CrossRef] [PubMed]
- Szabó, K.J.; Wendt, O.F. (Eds.) Pincer and Pincer-Type Complexes: Applications in Organic Synthesis and Catalysis; John Wiley & Sons: New York, NY, USA, 2014. [Google Scholar]
- Lawrence, M.A.W.; Green, K.-A.; Nelson, P.N.; Lorraine, S.C. Review: Pincer ligands—Tunable, versatile and applicable. Polyhedron 2018, 143, 11–27. [Google Scholar] [CrossRef]
- Yorke, J.; Sanford, J.; Decken, A.; Xia, A. Iminophosphinite pincer palladium complexes: Synthesis and application. Inorg. Chim. Acta 2010, 363, 961–966. [Google Scholar] [CrossRef]
- Sanford, J.; Dent, C.; Matsuda, J.D.; Xia, A. Synthesis, characterization and application of pincer-type nickel iminophosphinite complexes. Polyhedron 2011, 30, 1091–1094. [Google Scholar] [CrossRef]
- Yorke, J.; Beaton, S.; Jenkins, H.; Xia, A. Synthesis, Characterization and Applications of Novel Iminophosphinites. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 652–656. [Google Scholar] [CrossRef]
- Kadraoui, M.; Maunoury, T.; Derriche, Z.; Guillarme, S.; Saluzzo, C. Isohexides as Versatile Scaffolds for Asymmetric Catalysis. Eur. J. Org. Chem. 2015, 2015, 441–457. [Google Scholar] [CrossRef]
- Janvier, M.; Moebs-Sanchez, S.; Popowycz, F. Nitrogen-functionalized Isohexides in Asymmetric Induction. Chimia 2016, 70, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ejjiyar, S.; Saluzzo, C.; Amouroux, R.; Massoui, M. A direct single ring cleavage of isosorbide and isomannide with iodotrimethylsilane. Tetrahedron Lett. 1997, 38, 1575–1576. [Google Scholar] [CrossRef]
- Ejjiyar, S.; Saluzzo, C.; Massoui, M.; Amouroux, R.; Terry, N.; Coleman, A.W. Synthesis and assembly properties of a series of chiral amphiphilic dihydroxytetrahydrofuran derivatives. J. Phys. Org. Chem. 2001, 14, 1–10. [Google Scholar] [CrossRef]
- Ejjiyar, S. Obtention Stéréoselective de Cycles Tetrahydrofuranes à Partir de la Biomasse et Leur Ouverture Régioselective Pour la Synthèse de Molecules à Propriétés Spécifiques: Produits Naturels, Tensioactifs et Medicaments. Doctorate Thesis, IBN Tofail University, Kenitra, Marocco, 29 June 1999. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).