3,3’-(4,11-Bis(4-(trifluoromethyl)benzyl)-1,4,8,11-Tetraazacyclotetradecane-1,8-diyl)dipropanenitrile
Abstract
1. Introduction
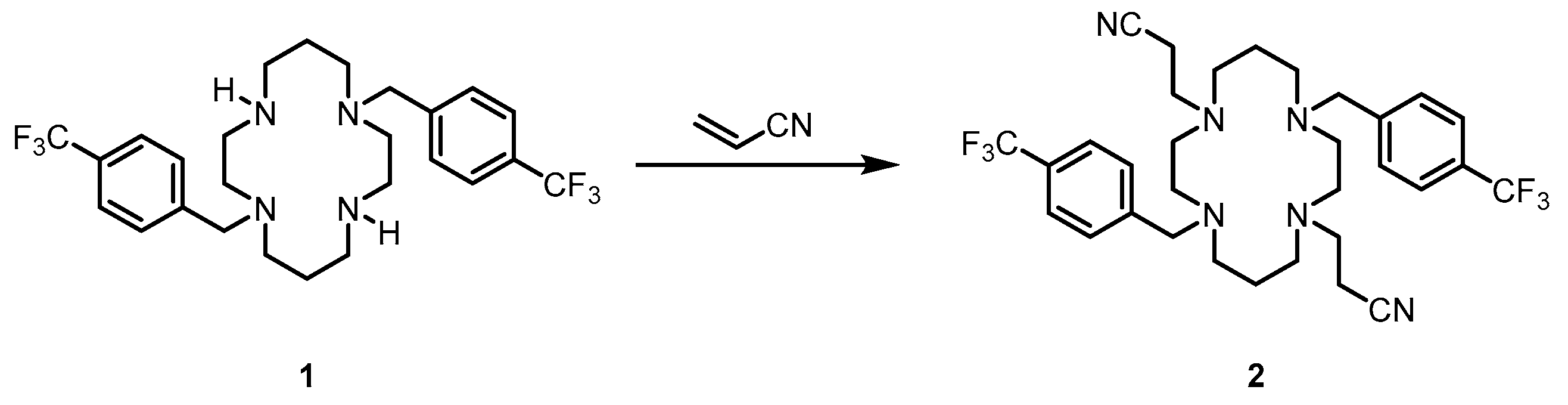
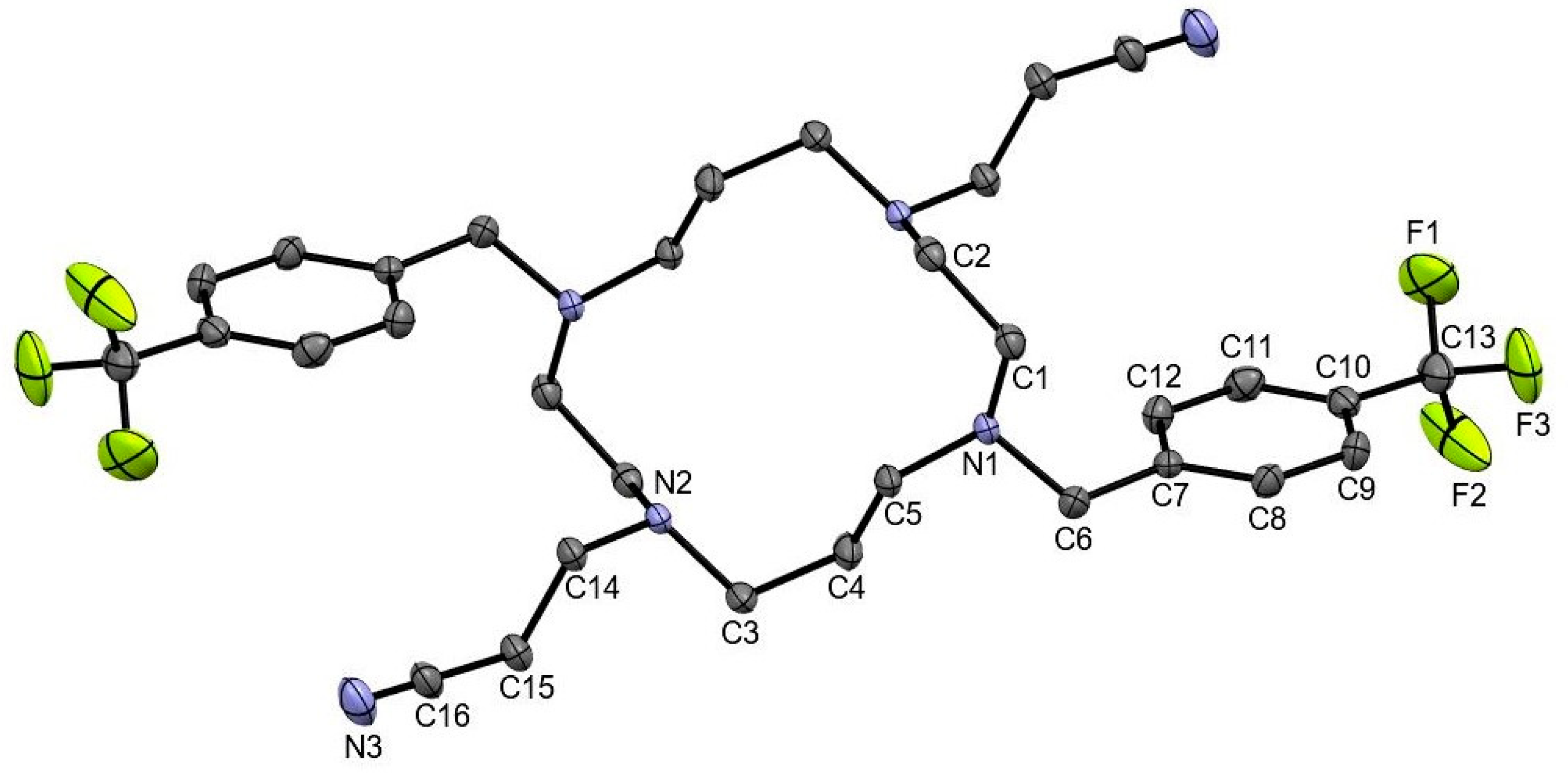
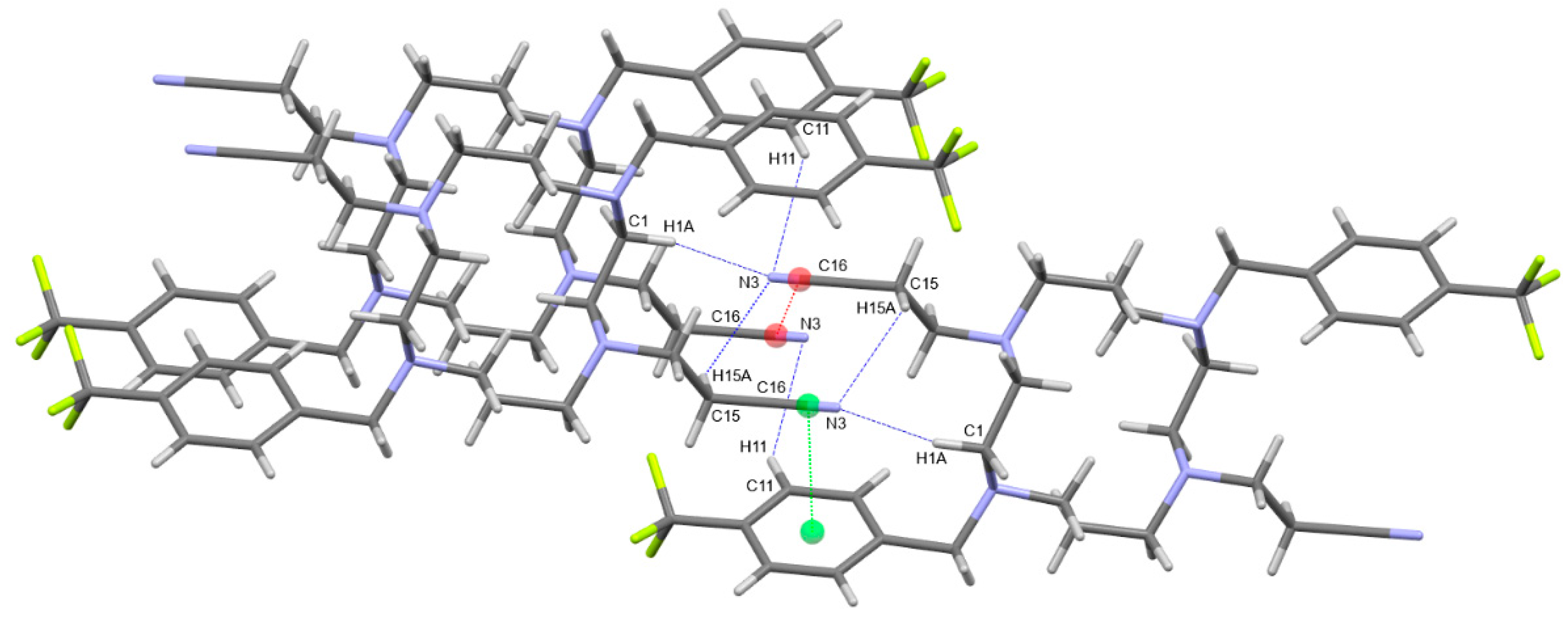
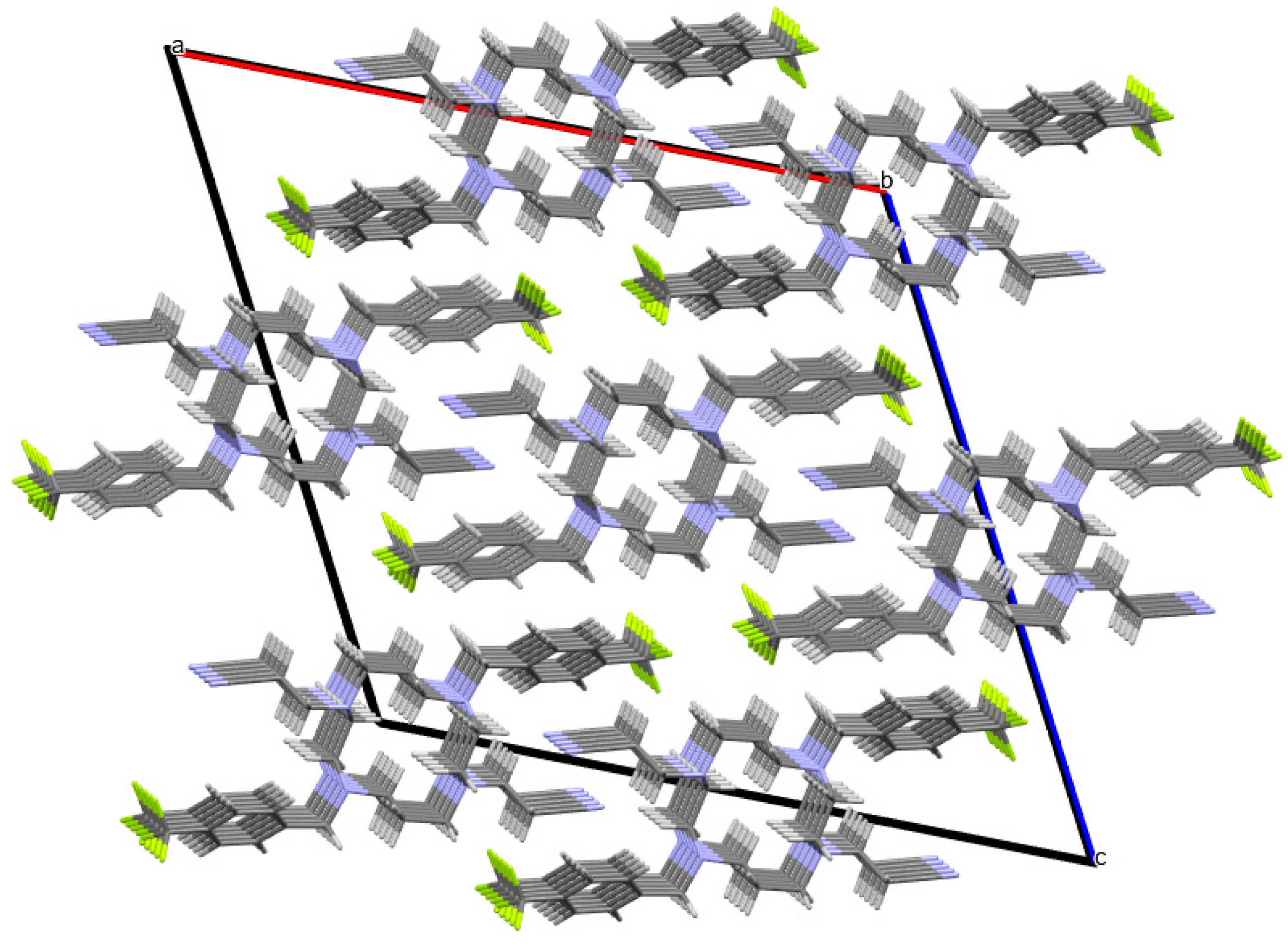
2. Results and Discussion
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis and Characterization
3.3. General Procedure for Single Crystal X-ray Crystallography
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mash, B.L.; Raghavan, A.; Ren, T. NiII Complexes of C-Substituted Cyclams Efficient Catalysts for Reduction of CO2 to CO. Eur. J. Inorg. Chem. 2019, 15, 2065–2070. [Google Scholar] [CrossRef]
- Alves, L.G.; Madeira, F.; Munhá, R.F.; Maulide, N.; Veiros, L.F.; Martins, A.M. Cooperative Metal-Ligand Hydroamination Catalysis Supported by C-H Activation in Cyclam Zr(IV) Complexes. Inorg. Chem. 2018, 57, 13034–13045. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Kwong, H.-K.; Lau, T.-C. Catalytic oxidation of water and alcohols by a robust iron(III) complex bearing a cross-bridged cyclam ligand. Chem. Commun. 2015, 51, 12189–12191. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.G.; Hild, F.; Munhá, R.F.; Veiros, L.F.; Dagorne, S. Synthesis and structural characterization of novel cyclam-based zirconium complexes and their use in the controlled ROP of rac-lactide: Access to cyclam-functionalized polylactide materials. Dalton Trans. 2012, 41, 14288–14298. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.A.; Munhá, R.F.; Alves, L.G.; Schafer, L.L.; Martins, A.M. Intramolecular hydroamination catalysis using trans-N,N’-dibenzylcyclam zirconium complexes. J. Organomet. Chem. 2011, 696, 2–6. [Google Scholar] [CrossRef]
- Alves, L.G.; Antunes, M.A.; Matos, I.; Munhá, R.F.; Duarte, M.T.; Fernandes, A.C.; Marques, M.M.; Martins, A.M. Reactivity of a new family of diamido-diamine cyclam-based zirconium complexes in ethylene polymerization. Inorg. Chimica Acta 2010, 363, 1823–1830. [Google Scholar] [CrossRef]
- Koucký, F.; Kotek, J.; Císařová, I.; Havlíčová, J.; Kubíček, V.; Hermann, P. Transition metal complexes of cyclam with 2,2,2-trifluoroethylphosphinate pendant arms for 19F magnetic resonance imaging. Dalton Trans. 2023, 52, 12208–12223. [Google Scholar] [CrossRef] [PubMed]
- Knighton, R.C.; Troadec, T.; Mazan, V.; Saëc, P.L.; Marionneau-Lambot, S.; Bihan, T.L.; Saffon-Merceron, N.; Bris, N.L.; Chérel, M.; Faivre-Chauvet, A.; et al. Cyclam-Based Chelators Bearing Phosphonated Pyridine Pendants for 64Cu-PET Imaging: Synthesis, Physicochemical Studies, Radiolabeling, and Bioimaging. Inorg. Chem. 2021, 60, 2634–2648. [Google Scholar] [CrossRef]
- Bihan, T.L.; Driver, C.H.S.; Ebenhan, T.; Bris, N.L.; Zeevaart, J.R.; Tripier, R. In Vivo Albumin-Binding of a C-Functionalized Cyclam Platform for 64Cu-PET/CT Imaging in Breast Cancer Model. ChemMedChem 2020, 16, 809–821. [Google Scholar] [CrossRef]
- Frindel, M.; Saëc, P.L.; Beyler, M.; Navarro, A.-S.; Saï-Maurel, C.; Alliot, C.; Chérel, M.; Gestin, J.-F.; Faivre-Chauvet, A.; Tripier, R. Cyclam te1pa for 64Cu PET imaging. Bioconjugation to antibody, radiolabeling and preclinical application in xerografted colorectal cancer. RSC Adv. 2017, 7, 9272–9283. [Google Scholar] [CrossRef]
- Blahut, J.; Bernášek, K.; Gálisová, A.; Herynek, V.; Císařová, I.; Kotek, J.; Lang, J.; Matějková, S.; Hermann, P. Paramagnetic 19F Relaxation Enhancement in Nickel(II) Complexes of N-Trifluoromethyl Cyclam Derivatives and Cell Labeling for 19F MRI. Inorg. Chem. 2017, 56, 13337–13348. [Google Scholar] [CrossRef] [PubMed]
- Blahut, J.; Hermann, P.; Gálisová, A.; Herynek, V.; Císařová, I.; Tošner, Z.; Kotek, J. Nickel(II) complexes of N-CH2CF3 cyclam derivatives as contrast agents for 19F magnetic resonance imaging. Dalton Trans. 2016, 45, 474–478. [Google Scholar] [CrossRef]
- Ali, M.; Chanda, M.; Oh, C.; Kohanim, S.; Mendez, R.; Kong, F.; Zhang, Y.; Mohan, S.; Kim, E.; Yang, D. Synthesis of Tc-99m cyclam-2-nitroimidazole: A probe for imaging tumor hypoxia. J. Nucl. Med. 2011, 52, 1606. [Google Scholar]
- Spain, M.; Wong, J.K.-H.; Nagalingam, G.; Batten, J.M.; Hortle, E.; Oehlers, S.H.; Jiang, X.F.; Murage, H.E.; Orford, J.T.; Crisologo, P.; et al. Antitubercular Bis-Substituted Cyclam Derivatives: Structure-Activity Relationships and in Vivo Studies. J. Med. Chem. 2018, 61, 3595–3608. [Google Scholar] [CrossRef] [PubMed]
- Grabchev, I.; Yordanova, S.; Vasileva-Tonkova, E.; Cangiotti, M.; Fattori, A.; Alexandrova, R.; Stoyanov, S.; Ottaviani, M.F. A novel benzofurazan-cyclam conjugate and its Cu(II) complex: Synthesis, characterization and in vitro cytotoxicity and antimicrobial activity. Dyes Pigment. 2016, 129, 71–79. [Google Scholar]
- Almada, S.; Maia, L.B.; Waerenborgh, J.C.; Vieira, B.J.C.; Mira, N.P.; Silva, E.; Cerqueira, F.; Pinto, E.; Alves, L.G. Cyclam-based iron(iii) and copper(ii) complexes: Synthesis, characterization and application as antifungal agents. New J. Chem. 2022, 46, 16764–16770. [Google Scholar] [CrossRef]
- Hubin, T.J.; Amoyaw, P.N.A.; Roewe, K.D.; Simpson, N.C.; Maples, R.D.; Freeman, T.N.C.; Cain, A.M.; Le, J.G.; Archibald, S.J.; Khan, S.I.; et al. Synthesis and antimalarial activity of metal complexes of cross-bridged tetraazamacrocyclic ligands. Bioorg. Med. Chem. 2014, 22, 3239–3244. [Google Scholar] [CrossRef] [PubMed]
- Hubin, T.J.; Walker, A.N.; Davilla, D.J.; Freeman, T.N.C.; Epley, B.M.; Hasley, T.R.; Amoyaw, P.N.A.; Jain, S.; Archibald, S.J.; Prior, T.J.; et al. Tetrazamacrocyclic derivatives and their metal complexes as antileishmanial leads. Polyhedron 2019, 163, 42–53. [Google Scholar] [CrossRef]
- Khan, M.O.F.; Keiser, J.; Amoyaw, P.N.A.; Hossain, M.F.; Vargas, M.; Le, J.G.; Simpson, N.C.; Roewe, K.D.; Freeman, T.N.C.; Hasley, T.R.; et al. Discovery of Antishistosomal Drug Leads Based on Tetraazamacrocyclic Derivatives and Their Metal Complexes. Antimicrob. Agents Chemother. 2016, 60, 5331–5336. [Google Scholar] [CrossRef]
- Pilon, A.; Lorenzo, J.; Rodriguez-Calado, S.; Adão, P.; Martins, A.M.; Valente, A.; Alves, L.G. New Cyclams and Their Copper(II) and Iron(III) Complexes: Synthesis and Potential Application as Anticancer Agents. ChemMedChem 2019, 14, 770–778. [Google Scholar] [CrossRef]
- Maria, L.; Sousa, V.R.; Santos, I.C.; Mora, E.; Marçalo, J. Synthesis and structural characterization of polynuclear divalent ytterbium complexes supported by a bis(phenolate) cyclam ligand. Polyhedron 2016, 119, 277–285. [Google Scholar] [CrossRef]
- Maria, L.; Santos, I.C.; Alves, L.G.; Marçalo, J.; Martins, A.M. Rare earth metal complexes anchored on a new dianionic bis(phenolate)dimethylamineCyclam ligand. J. Organomet. Chem. 2013, 728, 57–67. [Google Scholar] [CrossRef]
- Kobelev, S.A.; Averin, A.D.; Maloshitskaya, O.A.; Denat, F.; Guilard, R.; Beletskaya, I.P. Planar-Chiral Macrobicycles Comprising Cyclam Moiety. Macroheterocycles 2012, 5, 389–395. [Google Scholar] [CrossRef]
- Comba, P.; Linti, G.; Zajaczkowski-Fischer, M.; Zessin, T. Copper(II) and zinc(II) chemistry of a new hexadentate cyclam-based bis-di-2-pyridylmethanamine ligand. J. Incl. Phenom. Macrocycl. Chem. 2011, 71, 331–337. [Google Scholar] [CrossRef]
- Svobodová, I.; Havlíčková, J.; Plutnar, J.; Lubal, P.; Kotek, J.; Hermann, P. Metal Complexes of 4,11-Dimethyl-1,4,8,11-tetrazacyclotetradecane-1,8-bis(methylphosphonic acid)—Thermodynamic and Formation/Decomplexation Kinetic Studies. Eur. J. Inorg. Chem. 2009, 2009, 3577–3592. [Google Scholar] [CrossRef]
- Morfin, J.-F.; Tripier, R.; Baccon, M.L.; Handel, H. Bismuth(III) coordination to cyclen and cyclam bearing four appended groups. Polyhedron 2009, 28, 3691–3698. [Google Scholar] [CrossRef]
- Igarashi, K.; Nogami, T.; Ishida, T. Ferromagnetic coupling of copper(II) and nickel(II) complexes with a cyclam-based paramagnetic host. Polyhedron 2009, 28, 1672–1677. [Google Scholar] [CrossRef]
- Xue, G.; Bradshaw, J.S.; Dalley, N.K.; Savage, P.B.; Izatt, R.M. Synthesis of trans-disubstituted cyclam ligands appended with two 6-hydroxymethylpyridin-2-ylmethyl sidearms: Crystal structures of the 1,8-dimethyl-4,II-di(6-hydroxymethylpyridin-2-ylmethyl)cylam ligand and its Co(II) and Ni(II) complexes. J. Het. Chem. 2009, 40, 383–387. [Google Scholar] [CrossRef]
- Kotek, J.; Vojtíšek, P.; Císařová, I.; Hermann, P.; Jurečka, P.; Rohovec, J.; Lukeš, I. Bis(methylphosphonic Acid) Derivatives of 1,4,8,11-Tetraazacyclotetradecane (Cyclam). Crystal and Molecular Structures, and Solution Properties. Collect. Czech. Chem. Commun. 2000, 65, 1289–1316. [Google Scholar] [CrossRef]
- Alves, L.G.; Munhá, R.F.; Martins, A.M. Synthesis and structural characterization of N,N’,N’’,N’’’-tetrasubstituted cyclams. Chem. Het. Comp. 2021, 57, 871–874. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1948; p. 187. [Google Scholar]
- Hao, C.-J.; Zhang, Y.-H. 3,3′-(5,5,7,12,12,14-Hexamethyl-1,4,8,11-tetraazacyclotetradecane-1,8-diyl)dipropanonitrile methanol disolvate. Acta Cryst. 2010, E66, o1089. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.G.; Hazari, S.K.S.; Dey, B.K.; Miah, H.A.; Tiekink, E.R.T. trans-(3S,5S,10R,12R)-1,8-Bis(2-cyanoethyl)-C-meso-3,5,7,7,10,12,14,14-octamethyl-1,4,8,11-tetraazacyclotetradecane. Acta Cryst. 2001, E57, o524–o525. [Google Scholar] [CrossRef]
- Chakraborty, A.; Rabi, S.; Dey, L.; Palit, D.; Dey, B.K.; Tiekink, E.R.T.; Roy, T.G. Cadmium(II) compounds of the bis-cyanoethyl derivative (LCX) of Me8[14]aneC (LC): Characterization and antibacterialstudies. Heliyon 2022, 8, e09678. [Google Scholar] [CrossRef] [PubMed]
- SAINT, Version 7.03A; Bruker AXS Inc.: Madison, WI, USA, 1997.
- Sheldrick, G.M. SADABS, Software for Empirical Absorption Corrections; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; Caro, L.D.; Giacovazzo, C.; Polidori, G.; Spagna, R. SIR2004: An improved tool for crystal structure determination and refinement. J. Appl. Cryst. 2005, 38, 381–388. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure and refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, I.M.; Silva, E.R.; Alves, L.G. 3,3’-(4,11-Bis(4-(trifluoromethyl)benzyl)-1,4,8,11-Tetraazacyclotetradecane-1,8-diyl)dipropanenitrile. Molbank 2024, 2024, M1807. https://doi.org/10.3390/M1807
Nunes IM, Silva ER, Alves LG. 3,3’-(4,11-Bis(4-(trifluoromethyl)benzyl)-1,4,8,11-Tetraazacyclotetradecane-1,8-diyl)dipropanenitrile. Molbank. 2024; 2024(2):M1807. https://doi.org/10.3390/M1807
Chicago/Turabian StyleNunes, Inês M., Elisabete R. Silva, and Luis G. Alves. 2024. "3,3’-(4,11-Bis(4-(trifluoromethyl)benzyl)-1,4,8,11-Tetraazacyclotetradecane-1,8-diyl)dipropanenitrile" Molbank 2024, no. 2: M1807. https://doi.org/10.3390/M1807
APA StyleNunes, I. M., Silva, E. R., & Alves, L. G. (2024). 3,3’-(4,11-Bis(4-(trifluoromethyl)benzyl)-1,4,8,11-Tetraazacyclotetradecane-1,8-diyl)dipropanenitrile. Molbank, 2024(2), M1807. https://doi.org/10.3390/M1807







