Abstract
The reaction of a mixture of equimolar quantities of 2-acetylbenzofuran and (2,4,6-trichlorophenyl)hydrazine in ethanol containing concentrated hydrochloric acid (0.2 mL; 37%) as a catalyst under reflux for two hours yielded 1-(1-(benzofuran-2-yl)ethylidene)-2-(2,4,6-trichlorophenyl)hydrazine. The crude product was purified by crystallization using dimethylformamide to provide the title heterocycle in a 90% yield. The structure of the new heterocycle was confirmed through X-ray diffraction and spectral analyses.
1. Introduction
Benzofuran is a fundamental structure that occurs naturally in many compounds with various biological activities. Both synthetic and natural compounds that contain benzofuran fragments have exhibited exciting pharmaceutical and agrochemical activity [1,2,3,4,5]. Different research studies have demonstrated the potent biological activity of benzofuran compounds, including anti-tumor, antibacterial, anti-oxidative, and antiviral properties [6,7,8,9,10].
Hydrazones exhibit a variety of biological and pharmacological characteristics, including antimicrobial, anti-inflammatory, analgesic, antifungal, antitubercular, antiviral, anticancer, antiplatelet, antimalarial, anticonvulsant, cardioprotective, antihelmintic, antiprotozoal, antitrypanosomal, and antischistosomiasis properties [11,12,13]. In addition, hydrazones have a broad range of potential uses in the creation of sensor materials. They have been applied in the detection of fluoride ions, cyanide ions, heavy metals, and toxic gases [14,15,16,17,18].
Recently, we have explored the synthesis and structure elucidation of a range of new heterocycles [19,20,21]. Hybrid molecules, which contain different moieties with biological activities, have the potential for higher potency and lower harmful effects [22]. Based on the properties of both benzofurans and hydrazones, it was of interest to synthesize a new heterocycle containing both moieties for future assessment. The current study explores the synthesis of the new heterocycle, using a simple procedure, and its structure elucidation.
2. Results and Discussion
2.1. Synthesis of 3
The title heterocycle was synthesized as shown in Scheme 1. The process involved the reaction of equimolar amounts of 2-acetylbenzofuran (1) and 2,4,6-trichlorophenylhydrazine (2) in boiling ethanol (EtOH) containing concentrated hydrochloric acid (HCl, 0.2 mL, 37%) for 2 h. The resulting mixture was allowed to cool, and the solid formed was collected and recrystallized from dimethylformamide (DMF). The title heterocycle, 1-(1-(benzofuran-2-yl)ethylidene)-2-(2,4,6-trichlorophenyl)hydrazine (3), was obtained in a yield of 90% as crystals following crystallization.
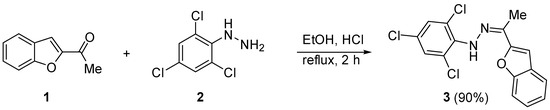
Scheme 1.
Synthesis of title heterocycle 3.
2.2. IR and NMR Spectroscopy of 3
The IR spectrum of hydrazone 3 showed an absorption band at 3355 cm–1 due to the NH group. The absorption bands for the C=C vibration in aromatic moieties appeared at 1613 and 1580 cm–1. The 1H NMR spectrum showed four singlet signals at 2.33, 7.15, 7.68, and 8.47 ppm, corresponding to the methyl, furyl, 2,4,6-trichlorophenyl, and NH protons, respectively. In addition, it showed multiple signals corresponding to the four protons of the benzofuran moiety. The 13C NMR spectrum showed two signals at low field (154.2 and 154.3 ppm) due to the C7a of the benzofuran moiety and the C=N carbon. The signal for C3 of the benzofuran moiety appeared at 104.4 ppm, and the methyl carbon was observed at 12.6 ppm. In addition, the spectrum showed all the other expected carbons (see Supplementary Materials for the spectra).
2.3. Crystal Structure of 3
The asymmetric unit of the crystal structure constitutes one molecule of 3 (Figure 1). The molecule is composed of three planar fragments, namely, benzofuran (bfn, C1–C8, O1), ethylidenehydrazine (edh, C9, C10, N1, N2), and trichlorophenyl (tcp, C11–C16, Cl1–Cl3) groups. In the molecule, the planes through the planar fragments are twisted relative to each other, with twist angles bfn/edh and edh/tcp of 8.11(13)° and 37.38(7)°, respectively. Intramolecular N–H…Cl contact is observed with an N2–H2B…Cl2 angle of 112(2)° and a N2…Cl2 distance of 2.939(2)°.
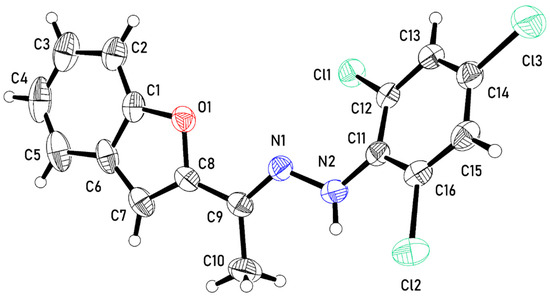
Figure 1.
An ortep representation of the asymmetric unit of 3 with atomic displacement parameters displayed at the 50% probability level.
The twist angles between the benzofuran and ethylidenehydrazine groups (bfn/edh) in the structure of 3 are similar to those observed in N′-[(1E)-1-(1-benzofuran-2-yl)ethylidene]pyridine-4-carbohydrazide [22], N′-[(1E)-1-(1-benzofuran-2-yl)ethylidene]pyridine-3-carbohydrazide [23], and N′-[1-(1-benzofuran-2-yl)ethylidene]-2-cyanoacetohydrazide [24], in which the angles are 23.44°, 15.28° and 13.52°, respectively. The angle between the planes through ethylidenehydrazine and trichlorophenyl groups (edh/tcp) is also comparable to that between ethylidenehydrazine and trichloro-3,5-difluorophenyl groups in (E)-2,3,5,6-tetrafluoro-4-{1-[2-(2,4,6-trichloro-3,5-difluorophenyl)hydrazinylidene]ethyl}aniline [25], in which the angle is 21.72°.
In the crystal structure of 3 (Figure 2a), π…π interactions occur between the benzofuran and trichlorophenyl groups of neighboring molecules, which are essentially parallel with a separation distance of ca. 3.4Å. The centroid-to-centroid distance of the two groups involved is 3.496A. These interactions form chains of molecules aligned parallel to the [101] direction (Figure 2b).
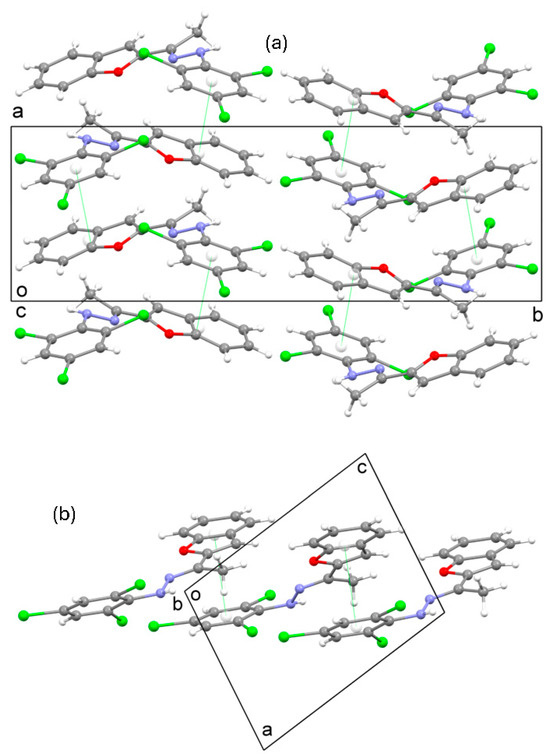
Figure 2.
(a) Crystal packing in the structure of 3 viewed down the c-axis and (b) a segment of the crystal structure showing a chain of molecules formed through π…π interactions. The π…π interactions are shown as green dotted lines.
3. Materials and Methods
3.1. General
Merck supplied the chemicals, reagents, and solvents. The Bruker Vertex 80 ATR-FTIR spectrometer (Bruker; Tokyo, Japan) was utilized to record the IR spectrum (400–4000 cm–1) of heterocycle 3. The NMR spectra were obtained in deuterated dimethyl sulfoxide (DMSO-d6) using a Varian Mercury 300 VX spectrometer (Varian, Palo Alto, CA, US) at 300 MHz for the protons and 75 MHz for the carbons. The chemical shift(δ) was reported in ppm, and the coupling constant (J) was measured in Hz. The preparation of 1 was based on a reported procedure [26].
3.2. Synthesis of 3
A mixture of 1 (0.32 g, 2.0 mmol) and 2 (0.42 g, 2.0 mmol) in EtOH (15 mL), containing HCl (0.2 mL; 37%), was refluxed for 2 h. After cooling down to 20 °C, the yellow solid obtained was filtered out. The product was washed with EtOH, dried, and finally recrystallized from DMF to yield 3 in a 90% yield as crystals. Mp 168–170 °C. IR (KBr): 3355, 2899, 1613, 1580 cm–1. 1H NMR: 2.33 (s, 3H, Me), 7.15 (s, 1H, furyl), 7.19–7.63 (m, 4H, Ar), 7.68 (s, 2H, 2,4,6-trichlorophenyl), 8.47 (s, 1H, NH). 13C NMR: 12.6, 104.4, 111.0, 121.2, 123.0, 124.8, 128.2, 128.8, 129.2, 130.7, 136.8, 138.3, 154.2, 154.3. Anal. Calcd. for C16H11Cl3N2O (351.99): C, 54.34; H, 3.14; N, 7.92. Found: C, 54.63; H, 3.29; N, 8.04%.
3.3. Crystal Structure Determination
An Agilent SuperNova Dual Atlas diffractometer (Rigaku, Tokyo, Japan) using mirror mono-chromated MoKα radiation was employed for data collection. The structure was solved with direct methods using SHELXT [27] and refined with SHELXL [28] using full-matrix least-squares methods on F2. MF = C16H11Cl3N2O, FW = 353.62, T = 293 (2) K, λ = 0.71073 Å, monoclinic, P21/n, a = 7.4396(5) Å, b = 22.2073(10) Å, c = 9.5228(5) Å, β = 100.766(6)°, V = 1545.60(15) Å3, Z = 4, calculated density = 1.520 Mg/m3, absorption coefficient = 0.594 mm−1, F (000) = 720, crystal size = 0.380 × 0.150 × 0.070 mm3, reflections collected = 14,110, independent reflections = 3873, R (int) = 0.0305, parameters = 204, goodness-of-fit on F2 = 1.021, R1 = 0.0428, wR2 = 0.0874 for (I > 2σ (I)), R1 = 0.0725, wR2 = 0.1030 for all data, and the largest difference peak and hole = 0.227 and −0.257 e.Å−3. The X-ray crystallographic data for heterocycle 3 have been deposited in the Cambridge Crystallographic Data Center with CCDC reference number 2334694.
4. Conclusions
The synthesis of a novel hydrazone with a benzofuran moiety has been carried out using a simple, convenient, and high-yielding procedure. The structure of the newly synthesized heterocycle has been established using nuclear magnetic resonance spectroscopy and X-ray diffraction techniques.
Supplementary Materials
The following are available online: IR, 1H, and 13C NMR spectra, CIFs, and CheckCIF reports for the title heterocycle 3.
Author Contributions
Conceptualization: G.A.E.-H. and B.M.K.; methodology: B.F.A.-W., B.M.K. and G.A.E.-H.; X-ray crystal structures: B.M.K.; investigation: B.F.A.-W., H.A.M., B.M.K. and G.A.E.-H.; writing—original draft preparation: B.F.A.-W., H.A.M., B.M.K. and G.A.E.-H.; writing—review and editing: B.F.A.-W., H.A.M., B.M.K. and G.A.E.-H. All authors have read and agreed to the published version of the manuscript.
Funding
National Research Centre and Cardiff and King Saud Universities.
Data Availability Statement
Data are contained within the article and the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Khodarahmi, G.; Asadi, P.; Hassanzadeh, F.; Khodarahmi, E. Benzofuran as a promising scaffold for the synthesis of antimicrobial and antibreast cancer agents: A review. J. Res. Med. Sci. 2015, 20, 1094–1104. [Google Scholar] [CrossRef]
- Dwarakanath, D.; Gaonkar, S.L. Advances in synthetic strategies and medicinal importance of benzofurans: A review. Asian J. Org. Chem. 2022, 11, e202200282. [Google Scholar] [CrossRef]
- Miao, Y.; Hu, Y.; Yang, J.; Liu, T.; Sun, J.; Wang, X. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019, 9, 27510–27540. [Google Scholar] [CrossRef] [PubMed]
- Khanam, H.; Shamsuzzaman. Bioactive benzofuran derivatives: A review. Eur. J. Med. Chem. 2015, 97, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Zadsirjan, V. Chapter Five-Recent Advances in the Synthesis of Benzo[b]furans. Adv. Heterocycl. Chem. 2015, 117, 261–376. [Google Scholar] [CrossRef]
- Chand, K.; Rajeshwari; Hiremathad, A.; Singh, M.; Santos, M.A.; Keri, R.S. A review on antioxidant potential of bioactive heterocycle benzofuran: Natural and synthetic derivatives. Pharmacol. Rep. 2017, 69, 281–295. [Google Scholar] [CrossRef]
- Kadieva, M.G.; Oganesyan, É.T. Methods for the synthesis of benzofuran derivatives (review). Chem. Heterocycl. Compd. 1997, 33, 1245–1258. [Google Scholar] [CrossRef]
- Dawood, K.M. An update on benzofuran inhibitors: A patent review. Expert Opin. Ther. Pat. 2019, 29, 841–870. [Google Scholar] [CrossRef]
- Arce-Ramos, L.; Castillo, J.-C.; Becerra, D. Synthesis and biological studies of benzo[b]furan derivatives: A review from 2011 to 2022. Pharmaceuticals 2023, 16, 1265. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, W.; Mou, C.; Zou, J.; Jin, Z.; Hao, G.; Chi, Y.R. Facile access to benzofuran derivatives through radical reactions with heteroatom-centered super-electron-donors. Nat. Commun. 2023, 14, 7381. [Google Scholar] [CrossRef]
- Verma, G.; Marella, A.; Shaquiquzzaman, M.; Akhtar, M.; Ali, M.R.; Alam, M.M. A review exploring biological activities of hydrazones. J. Pharm. Bioallied. Sci. 2014, 6, 69–80. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Carneiro Brum, J.; França, T.C.C.; LaPlante, S.R.; Villar, J.D.F. Synthesis and biological activity of hydrazones and derivatives: A review. Mini Rev. Med. Chem. 2020, 20, 342–368. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Jamwal, P.; Vaid, H.; Gurubrahamam, R. Synthesis of alkynyl hydrazones from unprotected hydrazine and their reactivity as diazo precursors. Org. Lett. 2023, 11, 1889–1894. [Google Scholar] [CrossRef]
- Ahmed, F.; Xiong, H. Recent developments in 1,2,3-triazole-based chemosensors. Dyes Pigm. 2021, 185, 108905. [Google Scholar] [CrossRef]
- Saini, N.; Wannasiri, C.; Chanmungkalakul, S.; Prigyai, N.; Ervithayasuporn, V.; Kiatkamjornwong, S. Furan/thiophene-based fluorescent hydrazones as fluoride and cyanide sensors. J. Photochem. Photobiol. A Chem. 2019, 385, 112038. [Google Scholar] [CrossRef]
- Aysha, T.S.; Mohamed, M.B.I.; El-Sedik, M.S.; Youssef, Y.A. Multi-functional colorimetric chemosensor for naked eye recognition of Cu2+, Zn2+ and Co2+ using new hybrid azo-pyrazole/pyrrolinone ester hydrazone dye. Dyes Pigm. 2021, 196, 109795. [Google Scholar] [CrossRef]
- Govindasamy, V.; Perumal, S.; Sekar, I.; Madheswaran, B.; Karuppannan, S.; Kuppannan, S.B. Phenothia-zine-thiophene hydrazide dyad: An efficient “on-off” chemosensor for highly selective and sensitive de-tection of Hg2+ ions. J. Fluoresc. 2021, 31, 667–674. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, L.; Liu, F.; Fan, Y.; Liu, Y.; Han, Y.; Hu, Y.; Su, J.; Song, C. Rapid and selective detection of aluminum ion using 1,2,3-triazole-4,5-dicarboxylic acid-functionalized gold nanoparticle-based colorimetric sensor. RSC Adv. 2021, 11, 30635–30645. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Mohamed, H.A.; Kariuki, B.M.; El-Hiti, G.A. (2Z,5Z)-5-((3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-2-((4-methoxyphenyl)imino)-3-phenylthiazolidin-4-one. Molbank 2023, 2023, M1665. [Google Scholar] [CrossRef]
- Kariuki, B.M.; Abdel-Wahab, B.F.; Mohamed, H.A.; Bekheit, M.S.; El-Hiti, G.A. Synthesis and characterization of novel 2-(1,2,3-triazol-4-yl)-4,5-dihydro-1H-pyrazol-1-yl)thiazoles and 2-(4,5-dihydro-1H-pyrazol-1-yl)-4-(1H-1,2,3-triazol-4-yl)thiazoles. Molecules 2022, 27, 8904. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Bekheit, M.S.; Kariuki, B.M.; El-Hiti, G.A. (E)-N’-(1-(Benzofuran-2-yl)ethylidene)-1-(4-methoxyphenyl)-5-methyl-1H-1,2,3-triazole-4-carbohydrazide. Molbank 2023, 2023, M1657. [Google Scholar] [CrossRef]
- Liman, W.; Ait Lahcen, N.; Oubahmane, M.; Hdoufane, I.; Cherqaoui, D.; Daoud, R.; El Allali, A. Hybrid molecules as potential drugs for the treatment of HIV: Design and applications. Pharmaceuticals 2022, 15, 1092. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Arshad, M.N.; Tahir, M.N.; Asiri, A.M.; Naseer, M.M.; Ishaq, M.; Khan, M.U.; Shafiq, Z. An efficient synthesis, structural (SC-XRD) and spectroscopic (FTIR, 1HNMR, MS spectroscopic) characterization of novel benzofuran-based hydrazones: An experimental and theoretical studies. J. Mol. Struct. 2000, 1216, 128318. [Google Scholar] [CrossRef]
- Alotaibi, M.H.; Mohamed, H.A.; Abdel-Wahab, B.F.; Hegazy, A.S.; Kariuki, B.M.; El-Hiti, G.A. Crystal structure of N’-(1-(benzofuran-2-yl)ethylidene)-2-cyanoacetohydrazide, C13H11N3O2. Z. Kristallogr.-New Cryst. Struct. 2019, 234, 361–362. [Google Scholar] [CrossRef]
- Politanskaya, L.; Bagryanskaya, I.; Tretyakov, E. Synthesis of polyfluorinated arylhydrazines, arylhydrazones and 3-methyl-1-aryl-1H-indazoles. J. Fluorine Chem. 2018, 214, 48–57. [Google Scholar] [CrossRef]
- Coşkun, D.; Tekin, S.; Sandal, S.; Coşkun, M.F. Synthesis, characterization, and anticancer activity of new benzofuran substituted chalcones. J. Chem. 2016, 2016, 7678486. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).