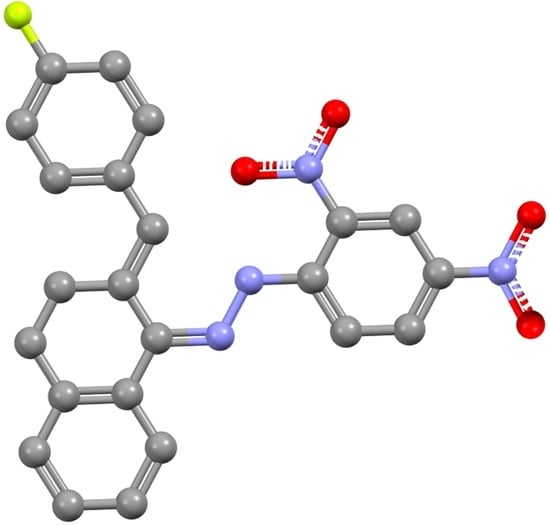
1-(2,4-Dinitrophenyl)-2-((Z)-2-((E)-4-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-ylidene)hydrazine
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of 3
2.2. IR and NMR Spectroscopy of 3
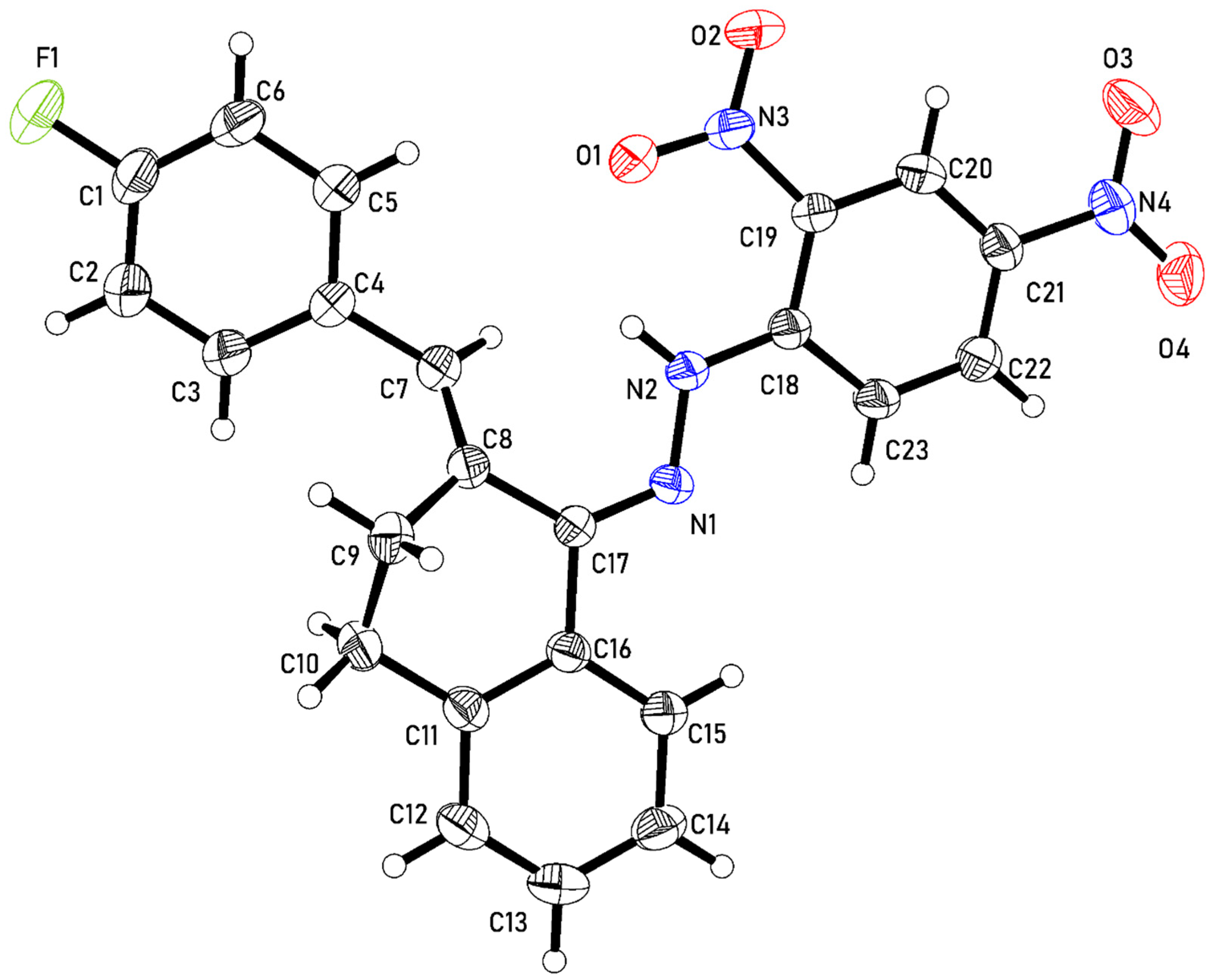
2.3. Crystal Structure of 3
3. Materials and Methods
3.1. General
3.2. Synthesis of 3
3.3. Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Oliveira Carneiro Brum, J.; França, T.C.C.; LaPlante, S.R.; Villar, J.D.F. Synthesis and biological activity of hydrazones and derivatives: A review. Mini-Rev. Med. Chem. 2020, 20, 342–368. [Google Scholar] [CrossRef]
- Verma, G.; Marella, A.; Shaquiquzzaman, M.; Akhtar, M.; Ali, M.R.; Alam, M.M. A review exploring biological activities of hydrazones. J. Pharm. Bioallied. Sci. 2014, 6, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, Ł. The application of hydrazones and hydrazide-hydrazones in the synthesis of bioactive azetidin-2-one derivatives: A mini review. Biomed. Pharmacother. 2023, 163, 114853. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.N.; Thorat, B.R.; Gaupta, D.R.; Pandey, A. Mini-review of the importance of hydrazides and their derivatives—Synthesis and biological activity. Eng. Proc. 2021, 11, 21. [Google Scholar] [CrossRef]
- Fallas, J.A.; González, L.; Corral, I. Density functional theory rationalization of the substituent effects in trifluoromethyl-pyridinol derivatives. Tetrahedron 2009, 65, 232–239. [Google Scholar] [CrossRef]
- Rollas, S.; Küçükgüzel, S.G. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Wahbeh, J.; Milkowski, S. The use of hydrazones for biomedical applications. SLAS Technol. 2019, 24, 161–168. [Google Scholar] [CrossRef]
- Biliz, Y.; Hasdemir, B.; Küçük, H.B.; Zaim, M.; Şentürk, A.M.; Kırmızıbekmez, A.M.; Kara, İ. Novel N-acyl hydrazone compounds as promising anticancer agents: Synthesis and molecular docking studies. ACS Omega 2023, 8, 20073–20084. [Google Scholar] [CrossRef]
- Iliev, I.; Kontrec, D.; Detcheva, R.; Georgieva, M.; Balacheva, A.; Galić, N.; Pajpanova, T. Cancer cell growth inhibition by aroylhydrazone derivatives. Biotechnol. Biotechnol. Equip. 2019, 33, 756–763. [Google Scholar] [CrossRef]
- Joule, J.A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons: Chichester, UK, 2010. [Google Scholar]
- Liaw, W.-F.; Lee, N.-H.; Chen, C.-H.; Lee, C.-M.; Lee, G.-H.; Peng, S.-M. Dinuclear and mononuclear iron(II)−thiolate complexes with mixed CO/CN- ligands: Synthetic advances for iron sites of [Fe]-only hydrogenases. J. Am. Chem. Soc. 2000, 122, 488–494. [Google Scholar] [CrossRef]
- Trotter, P.J.; White, P.A. Resonance raman determination of the triiodide structure in bis(tetrathiotetracene)triiodide organic conductor compared with the poly(vinyl alcohol)-iodine complex. Appl. Spectrosc. 1978, 32, 323–324. [Google Scholar] [CrossRef]
- Ahmed, F.; Xiong, H. Recent developments in 1,2,3-triazole-based chemosensors. Dyes Pigm. 2021, 185, 108905. [Google Scholar] [CrossRef]
- El-Nagar, I.; Youssef, A.M.; Abd El-Hakim, A.A.; Kenawy, E.-R.; Mandour, H.S.A.; Khattab, T.A. Novel hydrazone chromophore sensor for metallochromic determination of cadmium ions. Chemosensors 2022, 10, 451. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, L.; Liu, F.; Fan, Y.; Liu, Y.; Han, Y.; Hu, Y.; Su, J.; Song, C. Rapid and selective detection of aluminum ion using 1,2,3-triazole-4,5-dicarboxylic acid-functionalized gold nanoparticle-based colorimetric sensor. RSC Adv. 2021, 11, 30635–30645. [Google Scholar] [CrossRef]
- Amin, Z.; Rauf, T.; Jan, Q.; Kuchey, M.Y.; Sofi, F.A.; Ismail, T.; Rashid, A.; Bhat, B.A.; Sidiq, N.; Bhat, M.A. Synthesis of a novel hydrazone functionality based spectrophotometric probe for selective and sensitive estimation of toxic heavy metal ions. ChemistrySelect 2023, 8, e202202632. [Google Scholar] [CrossRef]
- Rupa, S.A.; Patwary, M.A.M.; Ghann, W.E.; Abdullahi, A.; Uddin, A.K.M.R.; Mahmud, M.M.; Haque, M.A.; Uddin, J.; Kazi, M. Synthesis of a novel hydrazone-based compound applied as a fluorescence turn-on chemosensor for iron(III) and a colorimetric sensor for copper(II) with antimicrobial, DFT and molecular docking studies. RSC Adv. 2023, 13, 23819–23828. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Bekheit, M.S.; Kariuki, B.M.; El-Hiti, G.A. (E)-N′-(1-(Benzofuran-2-yl)ethylidene)-1-(4-methoxyphenyl)-5-methyl-1H-1,2,3-triazole-4-carbohydrazide. Molbank 2023, 2023, M1657. [Google Scholar] [CrossRef]
- Kariuki, B.M.; Abdel-Wahab, B.F.; Mohamed, H.A.; Bekheit, M.S.; El-Hiti, G.A. Synthesis and characterization of novel 2-(1,2,3-triazol-4-yl)-4,5-dihydro-1H-pyrazol-1-yl)thiazoles and 2-(4,5-dihydro-1H-pyrazol-1-yl)-4-(1H-1,2,3-triazol-4-yl)thiazoles. Molecules 2022, 27, 8904. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Mohamed, H.A.; Kariuki, B.M.; El-Hiti, G.A. (2Z,5Z)-5-((3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-2-((4-methoxyphenyl)imino)-3-phenylthiazolidin-4-one. Molbank 2023, 2023, M1665. [Google Scholar] [CrossRef]
- Vásquez, G.B.; Reddy, G.; Gilliland, G.L.; Stevens, W.J. Dinitrobenzene induces methemoglobin formation from deoxyhemoglobin in vitro. Chem.-Biol. Interact. 1995, 96, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, Y.-Q.; Liu, Y.-K.; Zhang, H.-Q.; Hou, G.-G.; Meng, Q.-G.; Hou, Y. Potential anti-neuroinflammatory NF-κB inhibitors based on 3,4-dihydronaphthalen-1(2H)-one derivatives. J. Enzym. Inhib. Med. Chem. 2020, 35, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Haroon, M.; Akhtar, T.; Tahir, M.N. Crystal structure of (E)-2-(4-chlorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one: A second monoclinic polymorph. Acta Cryst. 2015, E71, o741–o742. [Google Scholar] [CrossRef] [PubMed]
- Danish, M.; Hamid, M.; Tahir, M.N.; Ahmad, N.; Ghafoor, S. 1-(2,4-Dinitrophenyl)-2-(1,2,3,4-tetrahydronaphthalen-1-ylidene)hydrazine. Acta Cryst. 2010, E66, o2100. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, S.; Shabanpoor, M.R.; Dehghani, Z.; Firuzi, O.; Edraki, N.; Khoshneviszadeh, M. Dihydronaphthalenone chalconoid derivatives as potential cathepsin B inhibitors; design, synthesis, cytotoxicity evaluation and docking analysis. Braz. J. Pharm. Sci. 2021, 57, e18074. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Wahab, B.F.; Mohamed, H.A.; Kariuki, B.M.; El-Hiti, G.A. 1-(2,4-Dinitrophenyl)-2-((Z)-2-((E)-4-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-ylidene)hydrazine. Molbank 2024, 2024, M1789. https://doi.org/10.3390/M1789
Abdel-Wahab BF, Mohamed HA, Kariuki BM, El-Hiti GA. 1-(2,4-Dinitrophenyl)-2-((Z)-2-((E)-4-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-ylidene)hydrazine. Molbank. 2024; 2024(1):M1789. https://doi.org/10.3390/M1789
Chicago/Turabian StyleAbdel-Wahab, Bakr F., Hanan A. Mohamed, Benson M. Kariuki, and Gamal A. El-Hiti. 2024. "1-(2,4-Dinitrophenyl)-2-((Z)-2-((E)-4-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-ylidene)hydrazine" Molbank 2024, no. 1: M1789. https://doi.org/10.3390/M1789
APA StyleAbdel-Wahab, B. F., Mohamed, H. A., Kariuki, B. M., & El-Hiti, G. A. (2024). 1-(2,4-Dinitrophenyl)-2-((Z)-2-((E)-4-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-ylidene)hydrazine. Molbank, 2024(1), M1789. https://doi.org/10.3390/M1789