Chlorido-pentamethylcyclopentadienyl-[2-(2-pyridyl-кN)-ferrocenyl-кC]-iridium(III)
Abstract
1. Introduction
2. Results
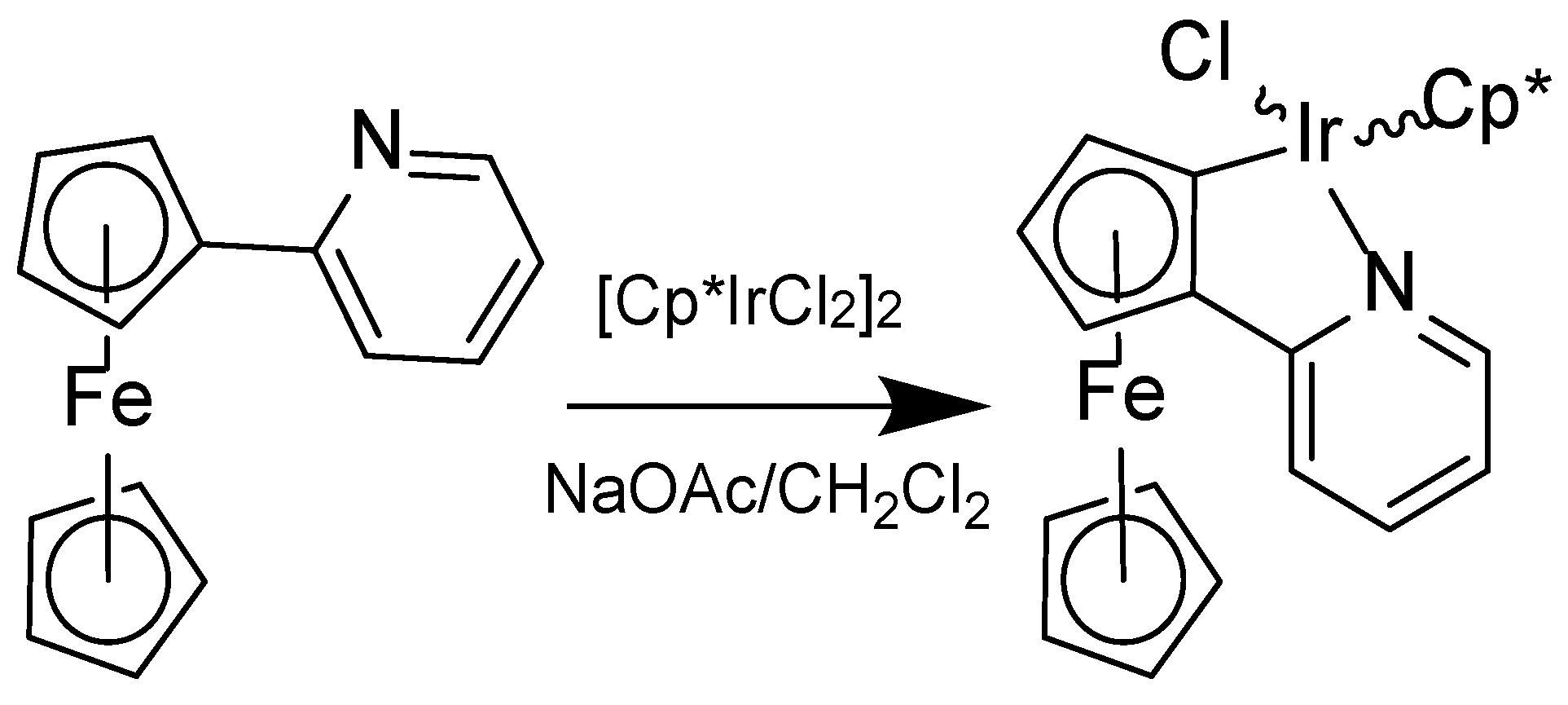
2.1. Synthesis and Spectroscopic Characterisation
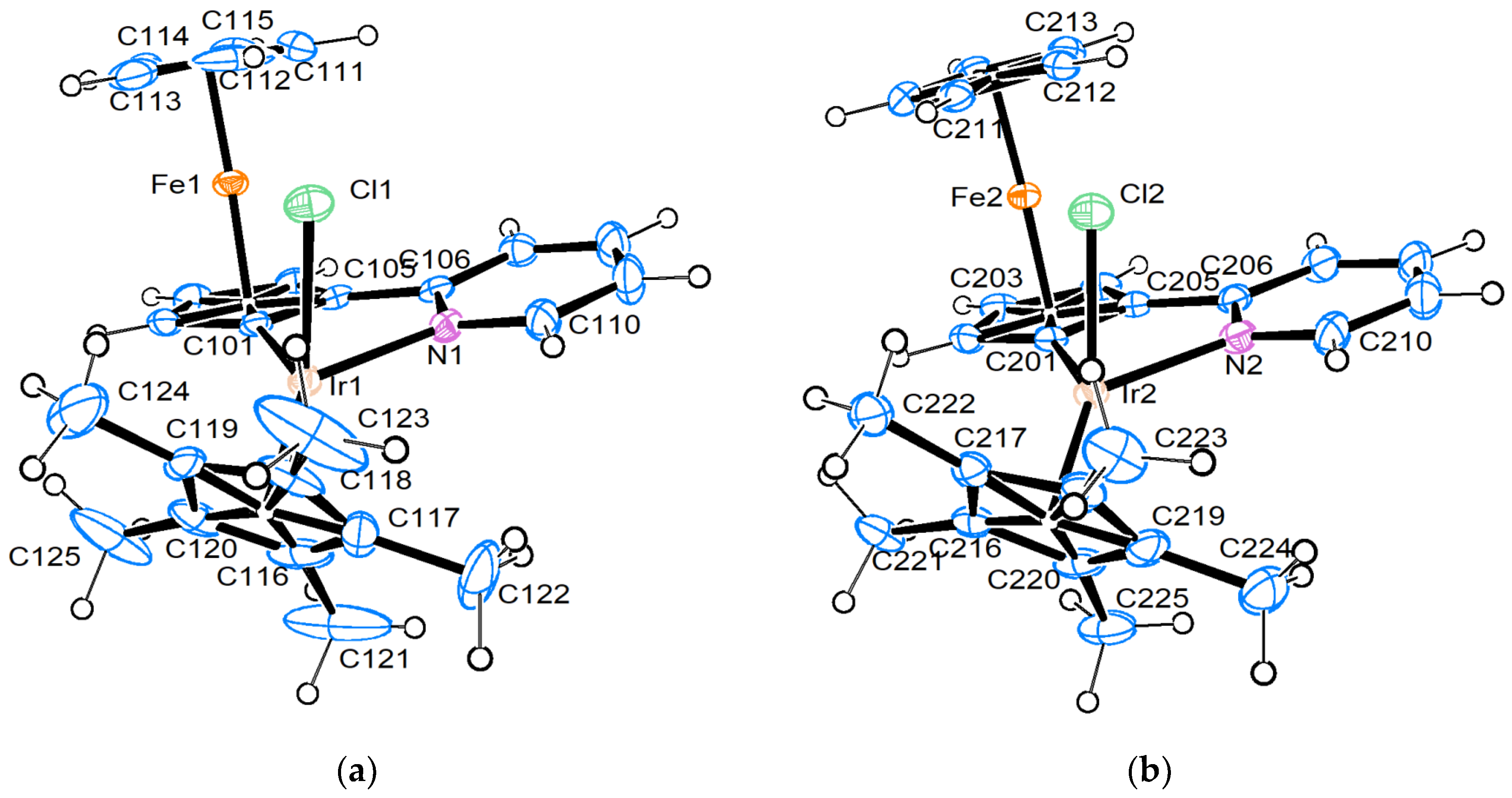
2.2. Crystallographic Study
3. Discussion
4. Materials and Methods
4.1. General Remarks
4.2. Synthesis
Chlorido-pentamethylcyclopentadienyl-[2-(2-pyridyl-кN)-ferrocenyl-кC]-iridium(III) (2)
4.3. Crystal Structure Determination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Sadler, P.J. Organoiridium Complexes: Anticancer Agents and Catalysts. Accounts Chem. Res. 2014, 47, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sadler, P.J. Redox-Active Metal Complexes for Anticancer Therapy. Eur. J. Inorg. Chem. 2017, 2017, 1541–1548. [Google Scholar] [CrossRef]
- Li, X.; Ouyang, W.; Nie, J.; Chen, Q.; Huo, Y. Recent Development on Cp*Ir(III)-Catalyzed C–H Bond Functionalization. ChemCatChem 2020, 12, 2358–2384. [Google Scholar] [CrossRef]
- Chen, Z.; Kacmaz, A.; Xiao, J. Recent Development in the Synthesis and Catalytic Application of Iridacycles. Chem. Rec. 2021, 21, 1506–1534. [Google Scholar] [CrossRef] [PubMed]
- Deaton, J.C.; Taliaferro, C.M.; Pitman, C.L.; Czerwieniec, R.; Jakubikova, E.; Miller, A.J.M.; Castellano, F.N. Excited-State Switching between Ligand-Centered and Charge Transfer Modulated by Metal–Carbon Bonds in Cyclopentadienyl Iridium Complexes. Inorg. Chem. 2018, 57, 15445–15461. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Wu, Y.; He, X.; Guo, X.; Gao, W.; Tan, L.; Yuan, X.-A.; Liu, J.; Liu, Z. In Vitro and In Vivo Antitumor Assay of Mitochondrially Targeted Fluorescent Half-Sandwich Iridium(III) Pyridine Complexes. Inorg. Chem. 2023, 62, 3395–3408. [Google Scholar] [CrossRef]
- Scheeren, C.; Maasarani, F.; Hijazi, A.; Djukic, J.-P.; Pfeffer, M.; Zaric, S.D.; LeGoff, X.-F.; Ricard, L. Stereoselective „Electrophilic“ Cyclometalation of Planar-Prochiral (η6-Arene)tricarbonylchromium Complexes with Asymmetric Metal Centers: Pseudo-T-4 [Cp*RhCl2]2 and [Cp*IrCl2]2. Organometallics 2007, 26, 3336–3345. [Google Scholar] [CrossRef]
- Djukic, J.-P.; Boulho, C.; Sredojevic, D.; Scheeren, C.; Zaric, S.; Ricard, L.; Pfeffer, M. The Stereospecific Ligand Exchange at a Pseudo-BenzylicT-4 Iridium Centre in Planar-Chiral Cycloiridium (η6-Arene)tricarbonylchromium Complexes. Chem. A Eur. J. 2009, 15, 10830–10842. [Google Scholar] [CrossRef]
- Djukic, J.-P.; Iali, W.; Pfeffer, M.; LeGoff, X.-F. Synthesis of Planar Chiral Iridacycles by Cationic Metal π-Coordination: Facial Selectivity, and Conformational and Stereochemical Consequences. Chem. Eur. J. 2012, 18, 6063–6078. [Google Scholar] [CrossRef]
- Rauf, U.; Shabir, G.; Bukhari, S.; Albericio, F.; Saeed, A. Contemporary Developments in Ferrocene Chemistry: Physical, Chemical, Biological and Industrial Aspects. Molecules 2023, 28, 5765. [Google Scholar] [CrossRef]
- Dwadnia, N.; Roger, J.; Pirio, N.; Cattey, H.; Hierso, J.-C. Input of P, N-(phosphanyl, amino)-ferrocene hybrid derivatives in late transition metals catalysis. Coord. Chem. Rev. 2018, 355, 74–100. [Google Scholar] [CrossRef]
- Ornelas, C.; Astruc, D. Ferrocene-Based Drugs, Delivery Nanomaterials and Fenton Mechanism: State of the Art, Recent Developments and Prospects. Pharmaceutics 2023, 15, 2044. [Google Scholar] [CrossRef]
- Ludwig, B.S.; Correia, J.D.; Kühn, F.E. Ferrocene derivatives as anti-infective agents. Coord. Chem. Rev. 2019, 396, 22–48. [Google Scholar] [CrossRef]
- Dai, L.; Xu, D.; Yang, M.-J. Synthesis of 2-oxazoline ferrocenes: Towards high-efficient chiral ligands and catalysis. J. Organomet. Chem. 2023, 999, 122831. [Google Scholar] [CrossRef]
- Liu, X.; Lv, A.; Zhang, P.; Chang, J.; Dong, R.; Liu, M.; Liu, J.; Huang, X.; Yuan, X.-A.; Liu, Z. The anticancer application of half-sandwich iridium(iii) ferrocene-thiosemicarbazide Schiff base complexes. Dalton Trans. 2023, 53, 552–563. [Google Scholar] [CrossRef]
- Shao, M.; Liu, X.; Sun, Y.; Dou, S.; Chen, Q.; Yuan, X.-A.; Tian, L.; Liu, Z. Preparation and the anticancer mechanism of configuration-controlled Fe(ii)–Ir(iii) heteronuclear metal complexes. Dalton Trans. 2020, 49, 12599–12609. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Chen, S.; Liu, X.; Wang, Q.; Gao, L.; Zhao, C.; Zhang, L.; Shao, M.; Yuan, X.-A.; Tian, L.; et al. Ferrocene-Appended Iridium(III) Complexes: Configuration Regulation, Anticancer Application, and Mechanism Research. Inorg. Chem. 2019, 58, 14175–14184. [Google Scholar] [CrossRef] [PubMed]
- Arthurs, R.A.; Horton, P.N.; Coles, S.J.; Richards, C.J. Phenyl vs. Ferrocenyl Cyclometallation Selectivity: Diastereoselective Synthesis of an Enantiopure Iridacycle. Eur. J. Inorg. Chem. 2016, 2017, 229–232. [Google Scholar] [CrossRef]
- Arthurs, R.A.; Horton, P.N.; Coles, S.J.; Richards, C.J. Metallocene to metallocene conversion. Synthesis of an oxazoline-substituted pentamethyliridocenium cation from a ferrocenyloxazoline. Chem. Commun. 2016, 52, 7024–7027. [Google Scholar] [CrossRef] [PubMed]
- Arthurs, R.A.; Ismail, M.; Prior, C.C.; Oganesyan, V.S.; Coles, S.J.; Richards, C.J. Enantiopure Ferrocene-Based Planar-Chiral Iridacycles: Stereospecific Control of Iridium-Centered Chirality. Chem. Eur. J. 2016, 22, 3065–3072. [Google Scholar] [CrossRef] [PubMed]
- Sünkel, K.; Branzan, R.; Weigand, S. 2-Pyridylmetallocenes, part IV. Cycloplatination of 2-pyridyl-ferrocene, -ruthenocene and –cymantrene. Molecular structures of σ-Pt{CpM[C5H3(2-C5H4N)]}Cl (DMSO) (M = Fe, Ru) and σ-Pt{CpFe[C5H3(2-C5H4N)]}(acac). Inorg. Chim. Acta 2013, 399, 193–199. [Google Scholar] [CrossRef]
- Sünkel, K.; Weigand, S. 2-Pyridylmetallocenes: Part 3 [1]. Cyclomercuration of 2-pyridyl-ferrocene and 1-(2-pyridyl)-2-methyl-ferrocene. Molecular structures of [1-(2-C5H4N)-2-(CH3)C5H3]Fe(C5H5), [1-(ClHg)-2-(2-C5H4N)C5H3]Fe(C5H5) and [1-(ClHg)-2-(2-C5H4N)-3-(CH3)C5H2]Fe(C5H5). Polyhedron 2012, 44, 133–137. [Google Scholar] [CrossRef]
- Weigand, S.; Sünkel, K. 2-Pyridylmetallocenes, Part VII. Synthesis and Reactivity of cycloaurated Pyridylmetallocenes: Spectroscopic and Crystallographic Characterization. Z. Anorg. Allg. Chem. 2024. submitted. [Google Scholar]
- Han, Y.-F.; Jin, G.-X. Cyclometalated [Cp*M(C^X)] (M= Ir, Rh; X = N, C, O, P) complexes. Chem. Soc. Rev. 2014, 43, 2799–2823. [Google Scholar] [CrossRef]
- Arthurs, R.A.; Hughes, D.L.; Horton, P.N.; Coles, S.J.; Richards, C.J. Application of Transmetalation to the Synthesis of Planar Chiral and Chiral-at-Metal Iridacycles. Organometallics 2019, 38, 1099–1107. [Google Scholar] [CrossRef]
- Arthurs, R.A.; Prior, C.C.; Hughes, D.L.; Oganesyan, V.S.; Richards, C.J. Enantiopure Planar Chiral and Chiral-at-Metal Iridacycles Derived fromBulky Cobalt Sandwich Complexes. Organometallics 2018, 37, 4204–4212. [Google Scholar] [CrossRef]
- Djukic, J.-P.; Hijazi, A.; Flack, H.D.; Bernardinelli, G. Non-racemic (scalemic) planar-chiral five-membered metallacycles: Routes, means, and pitfalls in their synthesis and characterization. Chem. Soc. Rev. 2007, 37, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Sünkel, K.; Weigand, S. 2-Pyridylmetallocenes: Part I. Electrophilic halogenation of 2-pyridylferrocene. Molecular structures of 2-pyridylferrocene and its a-brominated and-fluorinated derivatives. Synthesis of 2-pyridylruthenocene and 2-pyridylcymantrene. Inorg. Chim. Acta 2011, 370, 224–229. [Google Scholar] [CrossRef]
- Farrugia, L.J. WINGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
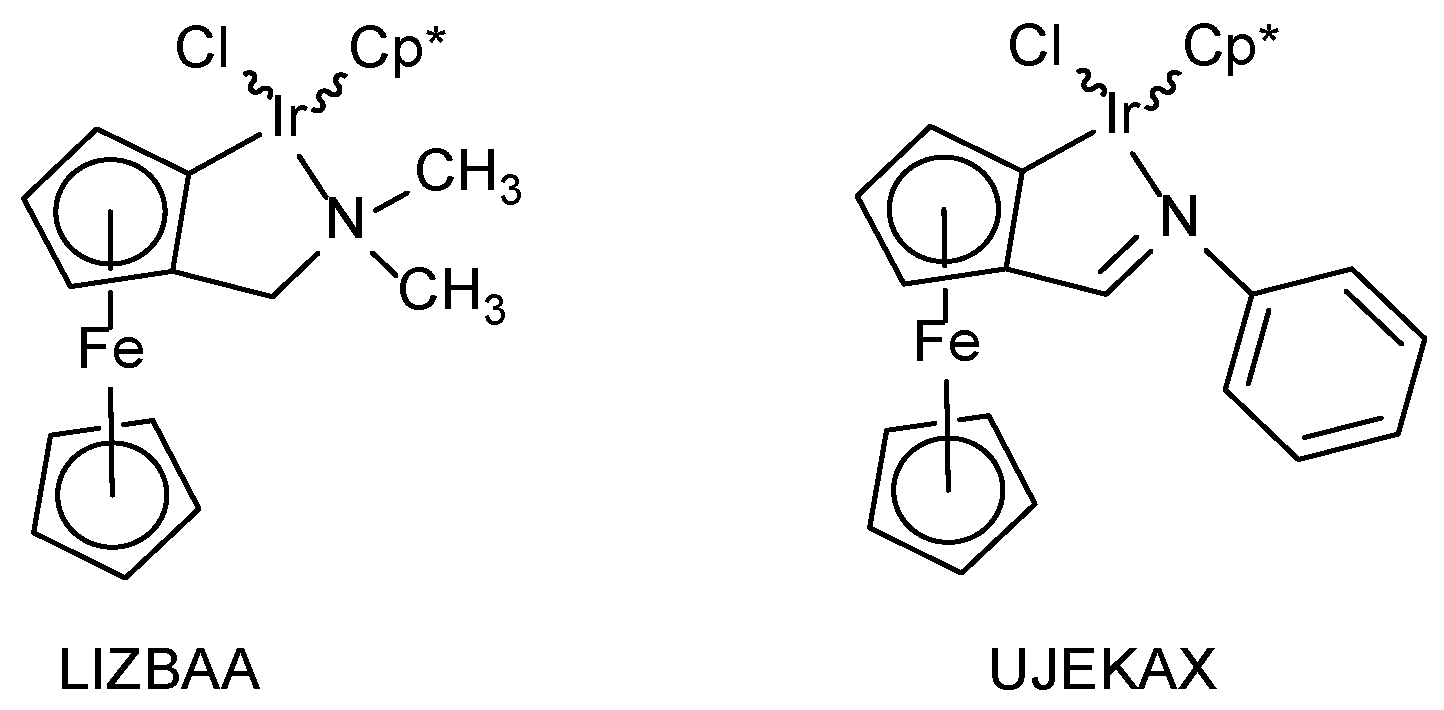
| Bond Length [Å] and Angles [°] 1 | Molecule A | Molecule B | LIZBAA 2 | UJEKAX 3 |
|---|---|---|---|---|
| Ir–Cl | 2.411(2) | 2.443(2) | 2.426(1) | 2.410(3) |
| Ir–N | 2.110(5) | 2.115(5) | 2.220(4) | 2.107(9) |
| Ir–CFc | 2.042(5) | 2.051(5) | 2.038(5) | 2.04(1) |
| Ir–CTcp* | 1.834(3) | 1.822(3) | 1.822 | 1.824 |
| Fe–CTcp,sub | 1.664(3) | 1.657(3) | 1.649 | 1.656 |
| Fe–CTC5H5 | 1.656(4) | 1.650(3) | 1.644 | 1.658 |
| CFc–Ir–N | 77.6(2) | 78.1(2) | 76.9(2) | 77.5(4) |
| CFc-CTcp,sub-CTC5H5-C’ | 13.1 | 21.1 | 10.3 | 17.3 |
| Fe…Cl | 4.036(2) | 3.968(2) | 3.850(1) | 5.077(3) |
| Fe…Ir | 3.901(1) | 3.832(1) | 3.8440(6) | 3.820(2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weigand, S.; Sünkel, K. Chlorido-pentamethylcyclopentadienyl-[2-(2-pyridyl-кN)-ferrocenyl-кC]-iridium(III). Molbank 2024, 2024, M1770. https://doi.org/10.3390/M1770
Weigand S, Sünkel K. Chlorido-pentamethylcyclopentadienyl-[2-(2-pyridyl-кN)-ferrocenyl-кC]-iridium(III). Molbank. 2024; 2024(1):M1770. https://doi.org/10.3390/M1770
Chicago/Turabian StyleWeigand, Stefan, and Karlheinz Sünkel. 2024. "Chlorido-pentamethylcyclopentadienyl-[2-(2-pyridyl-кN)-ferrocenyl-кC]-iridium(III)" Molbank 2024, no. 1: M1770. https://doi.org/10.3390/M1770
APA StyleWeigand, S., & Sünkel, K. (2024). Chlorido-pentamethylcyclopentadienyl-[2-(2-pyridyl-кN)-ferrocenyl-кC]-iridium(III). Molbank, 2024(1), M1770. https://doi.org/10.3390/M1770






