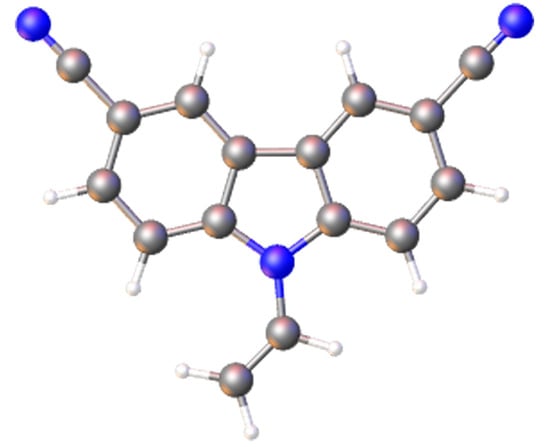
9-Vinyl-9H-carbazole-3,6-dicarbonitrile
Abstract
1. Introduction
2. Results
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis of 3,6-Dibromo-9H-carbazole 1
3.3. Synthesis of 9H-Carbazole-3,6-dicarbonitrile 2
3.4. 9-Vinyl-9H-carbazole-3,6-dicarbonitrile 3
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oner, S.; Bryce, M.R. A review of fused-ring carbazole derivatives as emitter and/or host materials in organic light emitting diode (OLED) applications. Mater. Chem. Front. 2023, 7, 4304–4338. [Google Scholar] [CrossRef]
- Srour, H.; Doan, T.-H.; Silva, E.D.; Whitby, R.J.; Witulski, B. Synthesis and molecular properties of methoxy-substituted diindolo[3,2-b:2′,3′-h]carbazoles for organic electronics obtained by a consecutive twofold Suzuki and twofold Cadogan reaction. J. Mater. Chem. C 2016, 4, 6270–6279. [Google Scholar] [CrossRef]
- Radhakrishna, K.; Manjunath, S.B.; Devadiga, D.; Chetri, R.; Nagaraja, A.T. Review on Carbazole-Based Hole Transporting Materials for Perovskite Solar Cell. ACS Appl. Energy Mater. 2023, 6, 3635–3664. [Google Scholar] [CrossRef]
- Carnes, J.E.; Warter, P.J. Photoinduced Current in Polyvinylcarbazole. Phys. Rev. B 1972, 5, 1557–1563. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, D.; Fang, C.; Li, Y. Mini-review on the novel synthesis and potential applications of carbazole and its derivatives. Des. Monomers Polym. 2023, 26, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Mittal, U.; Colasuonno, F.; Rawal, A.; Lessio, M.; Kundu, D. A highly stable 1.3 V organic cathode for aqueous zinc batteries designed in-situ by solid-state electrooxidation. Energy Storage Mater. 2022, 46, 129–137. [Google Scholar] [CrossRef]
- Mirle, C.R.; Raja, M.; Vasudevarao, P.; Sankararaman, S.; Kothandaraman, R. Functionalised carbazole as a cathode for high voltage non-aqueous organic redox flow batteries. New J. Chem. 2020, 44, 14401–14410. [Google Scholar] [CrossRef]
- Karon, K.; Lapkowski, M. Carbazole electrochemistry: A short review. J. Solid State Electrochem. 2015, 19, 2601–2610. [Google Scholar] [CrossRef]
- Walter, R.; Eugen, D. Production of Polymeric Nu-Vinyl Compounds. U.S. Patent US2072465, 10 July 1935. [Google Scholar]
- Jones, E.G.; Read, G. Photoconductivity and photovoltaism in polyvinylcarbazole membranes. Br. Polym. J. 2006, 14, 52–60. [Google Scholar] [CrossRef]
- Ellinger, L.P. Polyvinylcarbazole foams. II. Properties. J. Appl. Polym. Sci. 2003, 10, 575–600. [Google Scholar] [CrossRef]
- Aleshin, A.N.; Sokolovskaya, A.D.; Shcherbakov, I.P.; Brunkov, P.N.; Ulin, V.P. Organic light-emitting diodes based on polyvinylcarbazole films doped with polymer nanoparticles. Phys. Solid State 2013, 55, 675–680. [Google Scholar] [CrossRef]
- Jagt, J.C.; Sevriens, A.P.G. Electron sensitive negative resists of vinylaromatic polymers. Polym. Eng. Sci. 2004, 20, 1082–1086. [Google Scholar] [CrossRef]
- Bekkar, F.; Bettahar, F.; Moreno, I.; Meghabar, R.; Hamadouche, M.; Hernaez, E.; Vilas-Vilela, J.L.; Ruiz-Rubio, L. Polycarbazole and Its Derivatives: Synthesis and Applications. A Review of the Last 10 Years. Polymer 2020, 12, 2227. [Google Scholar] [CrossRef] [PubMed]
- Ergun, S.; Elliott, C.F.; Kaur, A.P.; Parkin, S.R.; Odom, S.A. Controlling Oxidation Potentials in Redox Shuttle Candidates for Lithium-Ion Batteries. J. Phys. Chem. C 2014, 118, 14824–14832. [Google Scholar] [CrossRef]
- Beletskii, E.V.; Fedorova, A.A.; Lukyanov, D.A.; Kalnin, A.Y.; Ershov, V.A.; Danilov, S.E.; Spiridonova, D.V.; Alekseeva, E.V.; Levin, O.V. Switchable resistance conducting-polymer layer for Li-ion battery overcharge protection. J. Power Sources 2021, 490, 229548. [Google Scholar] [CrossRef]
- Kulikov, I.; Panjwani, N.A.; Vereshchagin, A.A.; Spallek, D.; Lukianov, D.A.; Alekseeva, E.V.; Levin, O.V.; Behrends, J. Spins at work: Probing charging and discharging of organic radical batteries by electron paramagnetic resonance spectroscopy. Energy Environ. Sci. 2022, 15, 3275–3290. [Google Scholar] [CrossRef]
- Lukyanov, D.A.; Kalnin, A.Y.; Rubicheva, L.G.; Potapenkov, V.V.; Bakulina, O.Y.; Levin, O.V. Application of a TEMPO-Polypyrrole Polymer for NOx-Mediated Oxygen Electroreduction. Catalysts 2022, 12, 1466. [Google Scholar] [CrossRef]
- Wang, H.; Shao, Y.; Mei, S.; Lu, Y.; Zhang, M.; Sun, J.K.; Matyjaszewski, K.; Antonietti, M.; Yuan, J. Polymer-Derived Heteroatom-Doped Porous Carbon Materials. Chem. Rev. 2020, 120, 9363–9419. [Google Scholar] [CrossRef] [PubMed]
- Weishäupl, S.J.; Mayer, D.C.; Thyrhaug, E.; Hauer, J.; Pöthig, A.; Fischer, R.A. A nitrophenyl-carbazole based push-pull linker as a building block for non-linear optical active coordination polymers: A structural and photophysical study. Dye Pigment. 2021, 186, 109012. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svetlova, K.; Kazantsev, A.; Levin, O.; Filippova, S.; Alekseeva, E. 9-Vinyl-9H-carbazole-3,6-dicarbonitrile. Molbank 2024, 2024, M1768. https://doi.org/10.3390/M1768
Svetlova K, Kazantsev A, Levin O, Filippova S, Alekseeva E. 9-Vinyl-9H-carbazole-3,6-dicarbonitrile. Molbank. 2024; 2024(1):M1768. https://doi.org/10.3390/M1768
Chicago/Turabian StyleSvetlova, Kristina, Alexander Kazantsev, Oleg Levin, Sofia Filippova, and Elena Alekseeva. 2024. "9-Vinyl-9H-carbazole-3,6-dicarbonitrile" Molbank 2024, no. 1: M1768. https://doi.org/10.3390/M1768
APA StyleSvetlova, K., Kazantsev, A., Levin, O., Filippova, S., & Alekseeva, E. (2024). 9-Vinyl-9H-carbazole-3,6-dicarbonitrile. Molbank, 2024(1), M1768. https://doi.org/10.3390/M1768