1. Introduction
When synthesizing polycyclic fused ring systems, employing intramolecular cycloadditions can efficiently form two rings in a single synthetic step. Previous reports have used this strategy to their advantage. Meng and coworkers used the benzimidazole scaffold and intramolecular cycloaddition of azomethine ylides to prepare a series of pyrrolidino [2′,3′:3,4]pyrrolidino[1,2-
a]benzimidazoles [
1]. Benzimidazoles are prominent in many biologically active compounds and play an important role as therapeutic agents (e.g., antiulcer and anthelmintic drugs), in addition to displaying antimicrobial, antiviral, anticancer, anti-inflammatory, and analgesic activity [
2]. More recently, Villa and coworkers synthesized a series of fused pyrrolo[3,4-
b]quinoline compounds containing the benzimidazole core that were found to exhibit antifungal activity against pathogenic fungal strains [
3]. Cytotoxicity was not demonstrated against mammalian cells at the concentration that inhibited fungal growth [
3]. We became interested in using the benzimidazole scaffold as a precursor for hitherto unreported tetracyclic ring systems, especially using intramolecular cycloaddition. Benzimidazoles offer two sites for functionalization on the imidazole ring: at the N-1H and the C-2H.
Incorporating the isoxazole ring was of particular interest, as isoxazoles have unique functionality through their reductive ring-opened products and are readily prepared via [3 + 2] cycloaddition of a nitrile oxide and alkyne. One well-studied class of ring-opened isoxazole products is the β-enaminones, which can be readily prepared via reductive ring-opening through a number of methods (including catalytic hydrogenation with Pd/C or Raney Ni) [
4]. The resulting β-enaminone serves as a versatile precursor for various heterocycles, as exemplified by its recent application in synthesizing anti-inflammatory drugs such as celecoxib, deracoxib, and mavacoxib through the formation of pyrazoles from β-enaminones [
5]. A recent review has summarized reactions of β-enaminones [
6].
We present a novel tetracyclic ring system that incorporates a benzimidazole and an isoxazole, achieved by leveraging the substitution pattern of the benzimidazole and employing INOC methodology.
2. Results
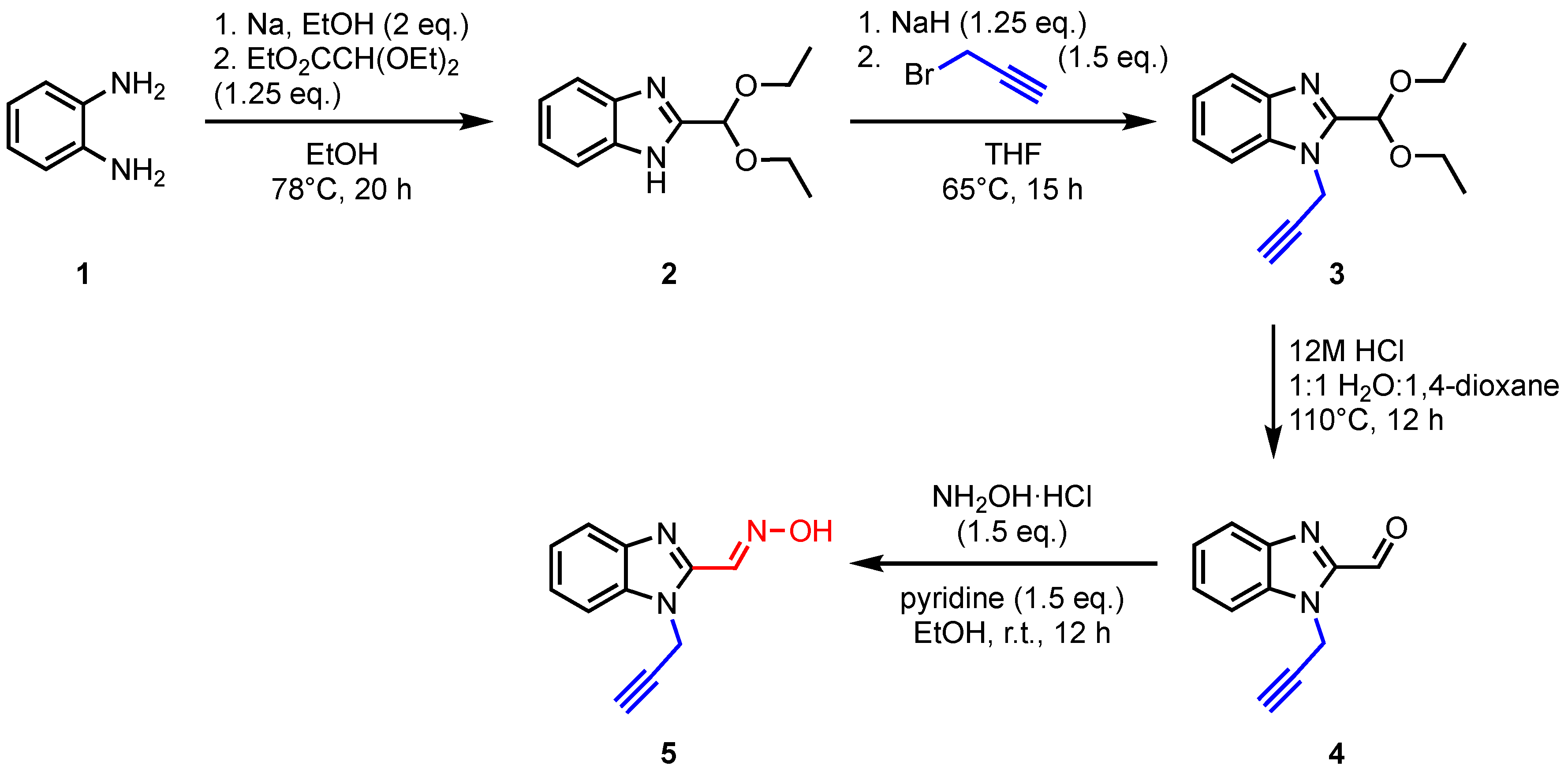
Using a benzimidazole scaffold having INOC in mind, we devised a synthetic scheme to afford aldoxime
5 based on the established synthesis of aldehyde
4 (
Scheme 1) [
1,
3].
In the same manner as in previous reports [
1,
3], the synthesis outlined in
Scheme 1 began with condensation of
o-phenylenediamine
1 with ethyl diethoxyacetate in refluxing ethanol. This formed benzimidazole
2 [
1,
7], which was deprotonated with NaH and alkylated with propargyl bromide in dry THF to yield
3. The acetal-bearing benzimidazole
3 was hydrolyzed by heating in strongly acidic conditions to afford aldehyde
4. Aldoxime
5 was then prepared from aldehyde
4 by reaction with hydroxylamine hydrochloride, using base to free hydroxylamine of its hydrochloride salt. Our goal when designing compound
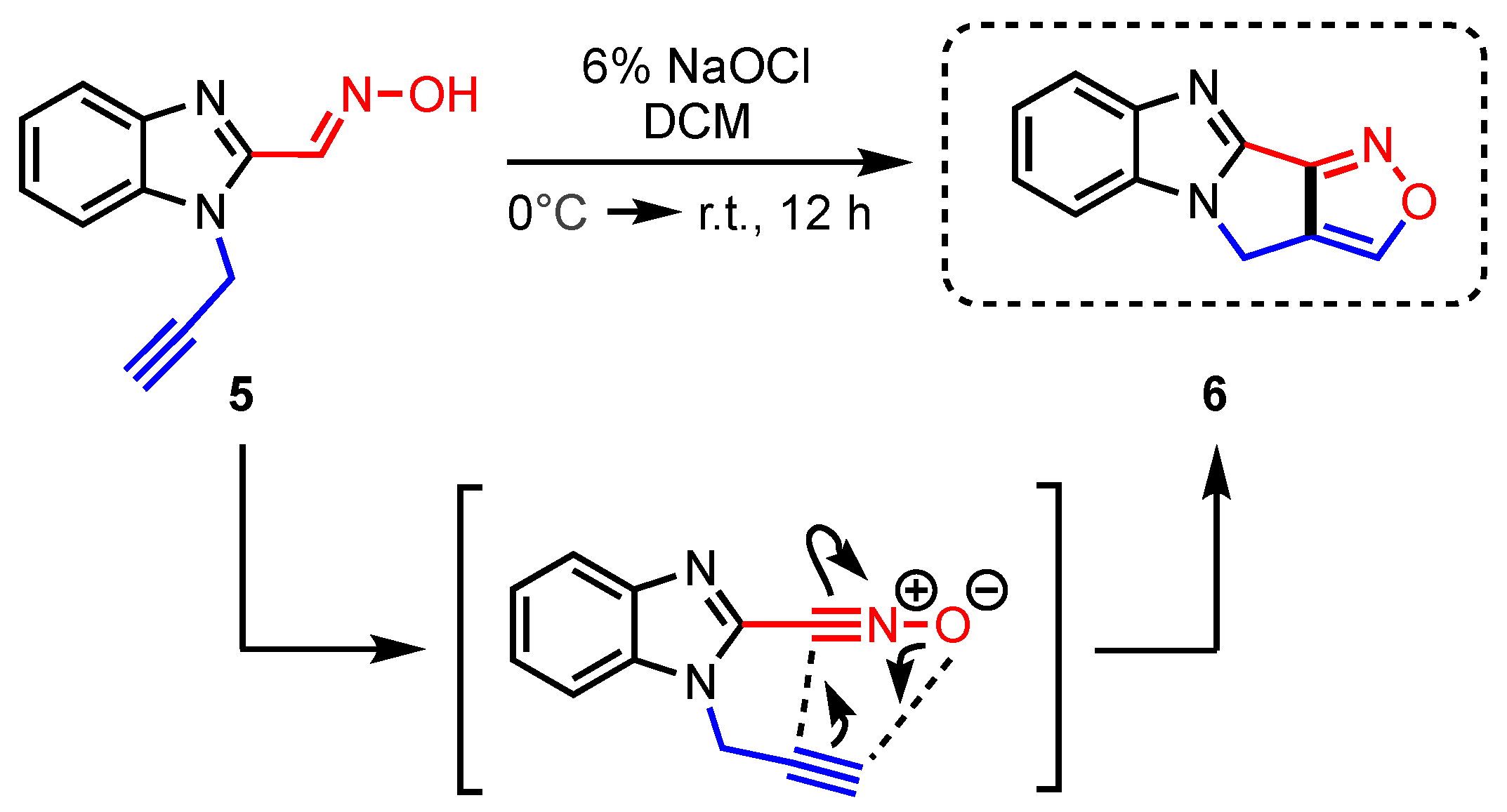
5 was to place a dipolarophile (the alkyne) adjacent to a 1,3-dipolar species (a nitrile oxide, easily generated in situ from the neighboring aldoxime). Indeed, a nitrile oxide intermediate was formed in situ from aldoxime
5 by stirring in biphasic bleach and DCM. The INOC reaction formed tetracyclic isoxazole
6 cleanly in 97% yield from
5 (
Scheme 2).
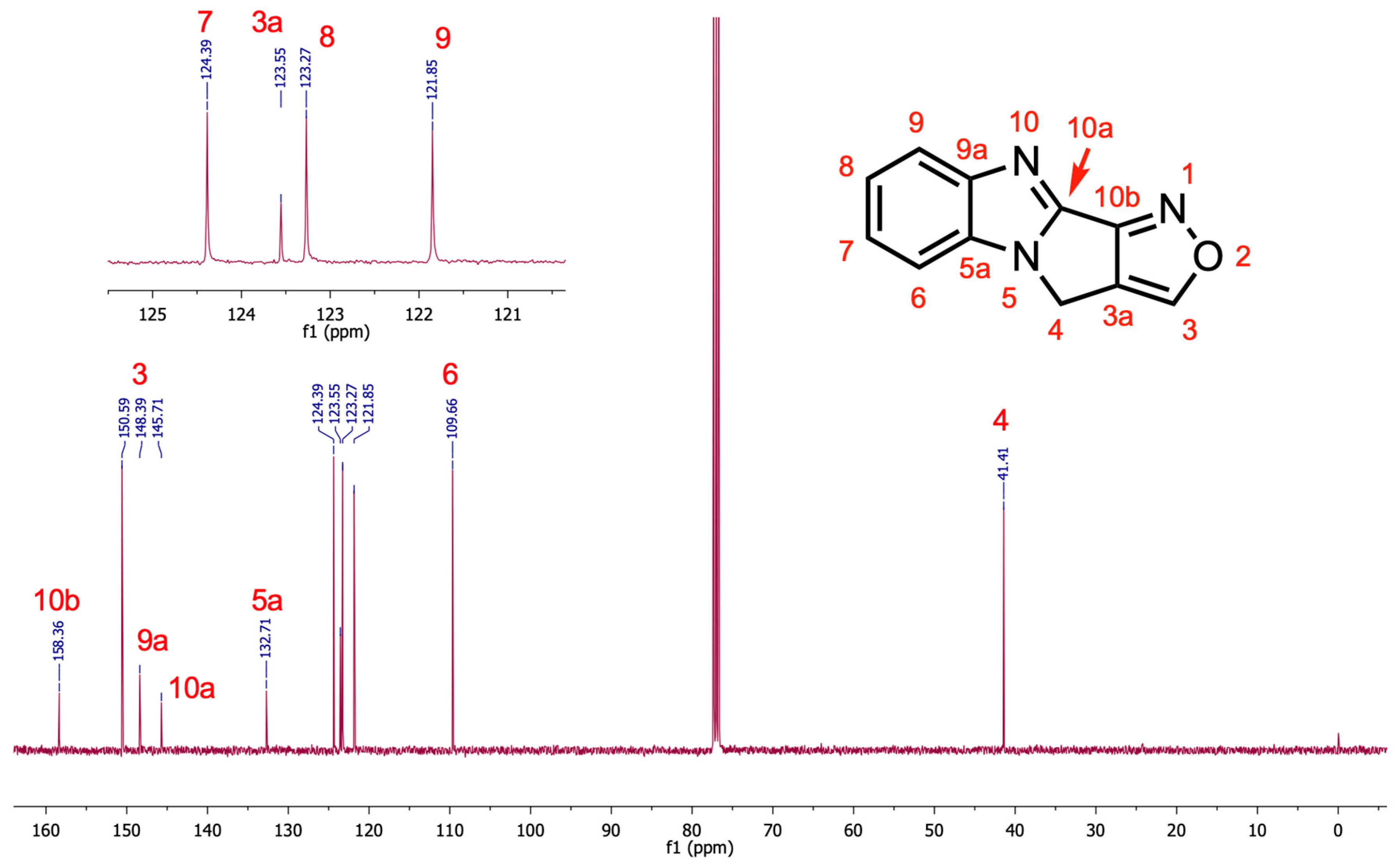
The 2D NMR spectra of
6 support assignment of the
13C-NMR signals. From the HMBC spectrum (see
Supplementary Material), the C-3H signal correlates with C-10b, C3a, and C-4. The C-4H signal correlates with C-5a, C-3a, C-3, C-10a, and C-10b. The C-6H signal correlates with C-5a and C-9a. The C-9H correlates with C-9a and C-5a. The annotated
13C-NMR spectrum is shown in
Figure 1.
The aromatic region of the
1H NMR spectrum (see
Supplementary Material) of
6 shows a singlet for the C-3H proton at δ 8.42, a doublet for the C-9H proton at δ 7.88, a doublet for the C-6H proton at δ 7.46, and a multiplet for the C-7H and C-8H protons at δ 7.35. A few additional noteworthy points can be made about compound
6. Because the alkyne and nitrile oxide moieties are effectively “locked in place” by the benzimidazole ring, cycloaddition between the two produces an isoxazole with a 3,4-substitution pattern. Isoxazoles of this substitution pattern can be challenging to synthesize, since under conventional conditions and due largely to steric and electronic effects, 1,3-dipolar [3 + 2] cycloadditions regioselectively form 3,5-disubstituted isoxazoles [
8]. This regioselectivity is often seen as an advantage, as mixtures of 3,5 and 3,4-isomers are avoided which would be time-consuming to separate. Despite this, an inherent byproduct of this convenience is difficulty obtaining 3,4-disubstiuted products. Isoxazoles with 3,4-substitution patterns can be prepared via complexation of the alkyne prior to cycloaddition, but an organoruthenium catalyst such as Cp*RuCl(COD) is needed [
9].
Interestingly, compound 6 is a novel ring system at the time of this publication, and rings bearing “6-5-5-5” ring fusions are rare in the current literature. Prior to this work, syntheses of a related antiaromatic “6-5-5-5” system was reported by Wang and coworkers [
10]. Conjugated polymers of a new class of π–expanded diketopyrrolopyrrole analogs demonstrated broad absorption in the visible region. The synthesis of a comparable “5-5-5” system was also reported [
11]. These accounts serve as precedents for this work, and the components of the reported “5-5-5” system correspond to the “5-5-5” structural section of
6.
3. Materials and Methods
All starting materials were purchased from commercially available sources and used as obtained. All reactions were performed in a ventilated hood. THF was distilled prior to use via a Na/ketyl still and stored over activated 4Å molecular sieves. Thin-layer chromatography (VWR International, Radnor, PA, USA) was performed on Agela Technologies aluminum-backed silica gel plates and the products were observed under 254 nm UV light. Flash chromatography was performed using Silicycle P60 silica gel (230–400 mesh). NMR spectra (400 MHz for 1H and 100 MHz for 13C, Bruker Biospin Corp., Billerica, MA, USA) were measured in CDCl3 or DMSO-d6. Chemical shifts (δ) were given in ppm relative to the resonance of their respective residual solvent peak, CHCl3 (7.27 ppm, 1H; 77.16 ppm, the middle peak, 13C). Multiplicities were described using the following abbreviations: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet. FT-IR experiments were performed using an ATR Perkin Elmer Spectrum 1 instrument (PerkinElmer Corp., Waltham, MA, USA) or Fisher Scientific Nicolet iS5 instrument (Thermo Fisher Scientific Co., Waltham, MA, USA). High-resolution mass-spectrometry (MS) experiments were recorded using an Applied Biosystems Voyager DE-STR MALDI-TOF (ABI) instrument (JBI Scientific, Huntsville, TX, USA).
3.1. Synthesis of 1-(2-propyn-1-yl)-1H-benzimidazole-2-aldoxime (5)
To a 50 mL round-bottomed flask was added 600 mg hydroxylamine hydrochloride (8.63 mmol, 1.5 eq.) along with a magnetic stir bar and 15 mL anhydrous EtOH. To this was added 692 μL (8.59 mmol, 1.5 eq.) pyridine. The solution was allowed to stir for 15 min. To this was then added 1.047 g 1-(2-propyn-1-yl)-1H-benzimidazole-2-carboxaldehyde (4) (5.26 mmol, 1 eq.) and the solution was stirred at r.t. for 12 h and monitored via TLC (1:1 EtOAc/hexanes). Upon completion, the solvent was removed under reduced pressure, 10 mL EtOAc added, and the undissolved brown solid vacuum filtered. The filtered solid was rinsed with EtOAc (3 × 10 mL) followed by deionized H2O (3 × 10 mL) and allowed to dry over vacuum yielding 952 mg 1-(2-propyn-1-yl)-1H-benzimidazole-2-aldoxime (5) in 84% yield. Purification could be carried out if needed by recrystallizing in anhydrous EtOH and rinsing the filtered solid with diethyl ether (3 × 10 mL). Tan solid, m.p.: 197–197.4 °C. (952 mg, 84%); 1H-NMR (DMSO-d6, 400 MHz) δ 3.38 (t, 1H, J = 2.49 Hz), δ 5.47 (d, 2H, J = 2.59 Hz), δ 7.30–7.38 (m, 2H, 7.04 Hz), δ 7.69 (t, 2H, 7.81 Hz), δ 8.31 (s, 1H), δ 12.21 (s, 1H); 13C-NMR (CDCl3, 100 MHz) δ 34.64, 75.84, 79.02, 111.18, 120.14, 123.17, 124.37, 135.81, 142.10, 142.92, 145.46. FT-IR (cm−1): 3300–3250 (w), 3250–2000 (br,w), 1004, 753 (s), 661; HRMS (TOF-MS ES+) m/z [M + H]+ calc. for C11H10N3O: 200.0824. Found: 200.0831.
3.2. Synthesis of Isoxazolo-4H-[3’,4’:3,4]pyrrolo [1,2-a]benzimidazole (6)
To a 150 mL round-bottomed flask was added 1.125 g (5.65 mmol, 1 eq.) 1-(2-propyn-1-yl)-1H-benzimidazole-2-aldoxime (5) followed by 65 mL DCM, which was vigorously stirred and cooled to 0 °C in an ice bath. To this was slowly added 20 mL of 6% aqueous NaOCl (6% commercial CloroxTM bleach) and 10 mL deionized H2O over the course of 15 min. The ice bath was removed, and the mixture was allowed to stir at ambient temperature for 12 h. The red solution was transferred to a separatory funnel and the aqueous layer extracted with DCM (3 × 10 mL). The combined DCM extracts were washed with brine (3 × 10 mL), dried over anhydrous Na2SO4, and the solvent removed under reduced pressure to give 1.090 g of 6 in 97% yield. Purification was carried via flash chromatography eluting with EtOAc/hexanes 4:1); TLC in EtOAc/hexanes 1:1, Rf = 0.37; tan solid, m.p.: 199.2–199.8 °C; 1H-NMR (CDCl3, 400 MHz) δ 5.05 (d, 2H, 1.07 Hz), δ 7.35–7.87 (m, 4H), δ 8.41 (s, 1H); 13C-NMR (CDCl3, 100 MHz) δ 41.41, 109.66, 121.85, 123.27, 123.55, 124.39, 132.71, 145.71, 148.39, 150.59, 158.36. FT-IR (cm−1): 3150–3110, 3075–3045, 1643, 1356, 838, 728; HRMS (TOF-MS ES+) m/z [M + H]+ calc. for C11H8N3O: 198.0667. Found: 198.0673.
4. Conclusions
The aforementioned work demonstrated the intramolecular 1,3-dipolar [3 + 2] cycloaddition of a nitrile oxide and alkyne to form two fused five-membered rings within a larger heterocyclic ring system. In total, a new ring system was created which places one six-membered ring fused to three heterocyclic five-membered rings (a “6-5-5-5” ring fusion pattern). There are few examples of this ring fusion pattern in the current literature, and this study suggests that ring strain need not play a significant role in the prevention of other ring systems with this fusion pattern. With respect to derivatization of
5 and
6, the starting diamine in the first synthetic step (
1), as well as the alkyne-containing bromide used in the second step, can both be varied to introduce molecular diversity. Various commercially available
o-phenylenediamines exist (such as dimethyl and various dihalide derivatives), and the synthesis is not limited to terminal alkynyl bromides. Internal alkynes can potentially lead to 3-substituted isoxazole moieties, which can ring-open to β-enaminones, known to serve as precursors to a number of other heterocycles [
12]. Broadly, because two rings are formed in a single step, INOC is shown to be an efficient tool to aid in the construction of polycyclic fused heterocyclic ring systems.