Abstract
N,N′-Dipropyloxamide (1) was synthesised by the reaction between diethyloxalate and n-propylamine in ethanol. Compound 1 was fully characterised by both microanalytical (elemental analysis, melting point determination) and spectroscopic means (FT-IR and NMR spectroscopy). Crystals suitable for single crystal X-ray diffraction were isolated by the slow evaporation of a methyl alcohol solution of the compound. The resulting crystal structure shows the prominent role exerted by intermolecular hydrogen bonds in the crystal packing.
1. Introduction
Derivatives of oxamic acid (oxalic acid monoamides) represent a family of compounds with applications in research fields that span from medicine [1,2] to organic synthesis [3,4] and cultural heritage [5,6,7,8,9]. Within this class of compounds, the oxalamide moiety (N,N′-diamide of oxalic acid) has been employed for the synthesis of biologically active compounds [10,11], precursors of widely used chemicals such as ethylene glycol [12,13], and ligands in coordination chemistry [14,15]. Moreover, oxalamides are self-complimentary hydrogen bonding molecules capable of donating and receiving two hydrogen bonds [16,17]. This property, combined with their persistent self-assembly behaviour, also allows oxalamide groups to participate in interesting hydrogen bonding (HB) motifs with applications in crystal engineering [18,19], protein engineering [20], organic gelators [21,22], and materials science [23].
Among N,N′-dialkyloxamides, N,N′-dipropyloxamide (1) has been employed as a precursor for N,N′-dialkylureas [24] and primary N-nitramines [25], and it has been studied by means of electronic spectroscopy [26,27], vibrational and NMR spectroscopy, thermal analysis, and ab initio calculations [28]. Despite this, the crystal structure of compound 1 was never reported, notwithstanding the importance and applications of the crystal packing interactions described above for this class of compounds, and the advantage of comparing the interactions in differently substituted derivatives.
We report here on the preparation and characterisation of N,N′-dipropyloxamide (1), and we describe for the first time its single-crystal XRD structural determination.
2. Results and Discussion
The synthesis of N,N′-disubstituted oxalamides is traditionally carried out by reacting oxalyl chloride and the desired amine [29,30], but more recently, N,N′-dialkyloxamide derivatives were also prepared by different methods, such as the catalytic carbonylation of amines [31]. We have instead synthesised compound 1 starting from diethyloxalate, which was reacted with n-propylamine in a 1:2 molar ratio, following the general method first described by Rice and co-workers in 1953 (Scheme 1) [32]. In particular, a mixture of the reagents in ethyl alcohol was refluxed under stirring overnight, and upon cooling down to room temperature, a white solid precipitated, which was isolated by filtration and recrystallised from methanol.
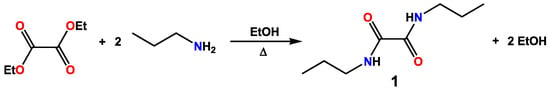
Scheme 1.
Synthetic pathway for the synthesis of compound 1.
Compound 1 was fully characterised by different means, including elemental analysis, melting point determination, FT-IR, 1H-, and 13C-NMR spectroscopy.
In the FT-IR spectrum of compound 1, the peak corresponding to the stretching mode of the N–H bond falls at 3300 cm–1, while the C=O stretching mode can be observed at 1653 cm–1. The peaks corresponding to the stretching modes of the C–H bonds in the aliphatic chain fall in the range 2850–3000 cm–1 (Figure S1). In the 1H-NMR spectrum recorded in CDCl3 for compound 1 (Figure S2), the signal corresponding to the N–H protons appears as a broad singlet at 7.50 ppm. On the other hand, the quadruplet corresponding to the -CH2- groups next to the nitrogen atoms falls at 3.27 ppm, and the signals of the remaining -CH2- and -CH3 groups of the alkyl chain are featured at 1.58 and 0.95 ppm, respectively. In the 13C{1H}-NMR spectrum of compound 1, also recorded in deuterated chloroform, the signal corresponding to the carbonyl groups resonates at around 160 ppm, while those of the remaining carbon atoms in the n-propyl chain fall between 11 and 42 ppm (Figure S3).
Compound 1 was recrystallised through the slow evaporation of a concentrated methanol solution of the compound, leading to the formation of colourless needle-shaped crystals suitable for single-crystal X-ray diffraction analysis. The compound crystallises in the triclinic P-1 space group (Tables S1–S4), the asymmetric unit containing half of the molecule (Figure 1). The carbonyl moiety showed the common antiperiplanar orientation with an inversion centre located halfway between the sp2-hybridised carbon atoms, making the oxalamide unit completely planar (Table S4). Previous studies demonstrated theoretically that the antiperiplanar conformation is indeed a minimum in the potential energy surface (PES) of N,N′-disubstituted oxalamides [9]. An examination of 258 different N,N′-disubstituted oxamides found in the Cambridge Structural Database (CSD) [33] showed average distances of 1.533, 1.226, and 1.328 Å for the C–C, C=O, and C–N bonds, respectively; very similar values were found in compound 1, the C4–C4i bond length amounting to 1.5366(18) Å, with a C4–O1 distance of 1.2370(11) Å, and a C4–N1 bond length of 1.3298(11) Å (Table S2).
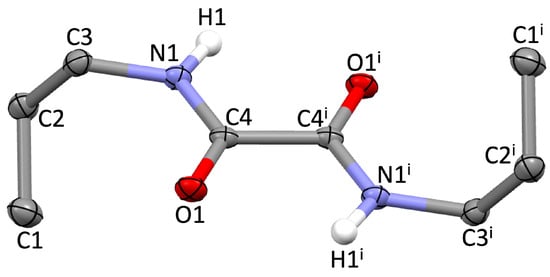
Figure 1.
Molecular structure and atom labelling scheme for compound 1. Thermal ellipsoids are drawn at 50% probability level; hydrogen atoms other than those of the N–H groups were omitted for clarity; i = 2–x, 1–y, 1–z.
The two n-propyl chains at the nitrogen atoms are on opposite sides of the oxamide unit and feature N1–C3–C2–C1 torsion angles of 68.91(11)° (Table S4).
As expected, the crystal packing of compound 1 is governed by N–H···O HB interactions. In particular, neighbouring molecules whose oxamide units lie on the same plane interact through HB motifs [34,35] running along the b-axis (Figure 2A, Table 1). These interactions are similar to those observed for other oxalamides featuring alkyl [17,28,36,37] or aryl [38,39,40] substituents.
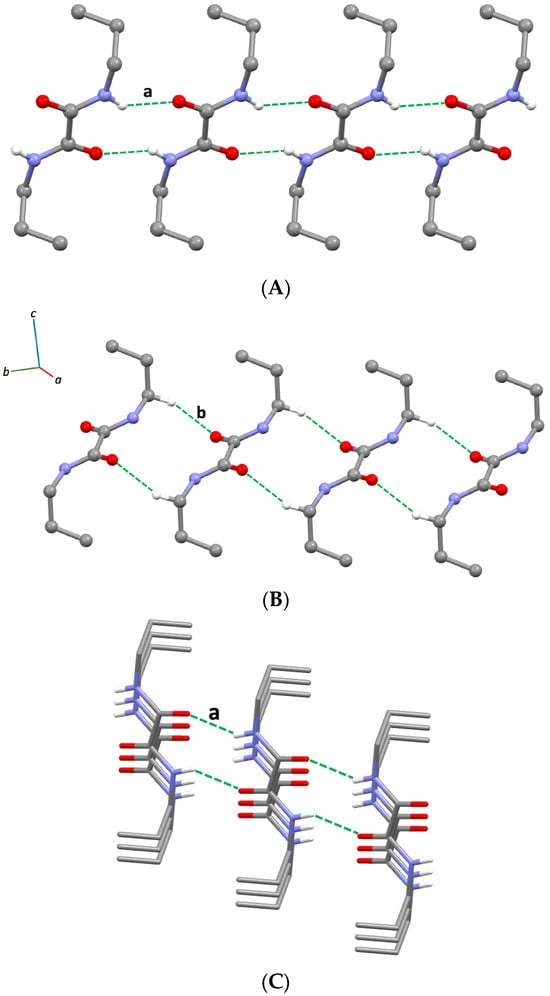
Figure 2.
(A) View of the crystal packing of compound 1 along the a-axis, showing the intermolecular N–H···O hydrogen bonding (HB) interactions (a, Table 1); (B) partial view of the crystal packing of compound 1 showing intermolecular C–H···O interactions (b, Table 1); (C) view of the crystal packing of compound 1 along the b-axis, showing the stacking of ribbons formed through HB interactions; only hydrogen atoms involved in intermolecular interactions are shown for clarity.

Table 1.
Intermolecular interactions of compound 1.
The ribbons formed by the described HBs along the b-axis are joined by C–H···O intermolecular interactions connecting the oxygen atoms at the carbonyl groups from one ribbon to the -CH2- groups next to the nitrogen atoms of another ribbon (Figure 2B, Table 1), leading to a stacking perpendicular to the b-axis (Figure 2C).
The described crystal packing is very similar to that recently described for the analogous compound N,N′-dibutyloxamide [41].
3. Materials and Methods
3.1. General Methods
Solvents and reagents were obtained from TCI, FluoroChem, and Merck and were employed without further purification. Deuterated solvents were obtained from Eurisotop and stored over molecular sieves prior to use. FT-IR measurements were recorded at room temperature on a Thermo-Nicolet 5700 spectrometer on KBr pellets by using a KBr beam splitter and KBr windows (4000−400 cm−1, resolution 4 cm−1). 1H- and 13C-NMR spectra were recorded in CDCl3 at room temperature on a Bruker Avance III HD 600 spectrometer. Chemical shifts are reported in ppm (δ). The residual 1H and 13C peaks of the deuterated solvent (CDCl3) were used for chemical shift calibration. Elemental analysis was performed with a CHNS/O PE 2400 series II elemental analyser (T = 925 °C). Uncorrected melting points were determined in capillaries on a FALC mod. C melting point apparatus.
3.2. X-ray Diffraction Analysis
X-ray diffraction data for compound 1 were collected at 100(2) K on a Rigaku FRE+ diffractometer, equipped with VHF Varimax confocal mirrors, an AFC12 goniometer, and a HyPix 6000 detector. The structure was solved with the ShelXT [42] solution program using dual methods, and the model was refined with ShelXL 2014/7 [43] using full matrix least squares minimisation on F2. Olex2 1.5 [44] was employed as the graphical interface.
3.3. Synthesis of N,N′-Dipropyloxamide (1)
Compound 1 was synthesised by reacting 1.22 g of n-propylamine (20.6 mmol) dissolved in 5 mL of ethanol with 1.50 g of diethyloxalate (10.3 mmol) diluted in 5 mL of the same solvent. The resulting mixture was refluxed under stirring overnight and then allowed to cool down to room temperature, resulting in the precipitation of a white solid. The solid was isolated by filtration on a Gooch funnel, washed with n-hexane, and air dried. The purity of the isolated product was confirmed by TLC (stationary phase: silica gel; eluent: dichloromethane/ethyl acetate 2:1). Recrystallisation by slow evaporation of a methanol solution of the product afforded colourless needle-shaped crystals suitable for XRD analysis (1.26 g; 7.3 mmol; Y = 71%). M. p. = 161.8 °C (Lit.: 162.5–163.5 °C [45]). Elemental analysis calcd (%) for C8H16N2O2: C 55.79, H 9.36, N 16.27. Found: C 55.26, H 9.32, N 16.35. FT-IR (KBr, 4000–400 cm−1): 3300 s, 3059 w, 2964 m, 2931 m, 2873 m, 1649 vs, 1529 m, 1460 w, 1439 w, 1383 w, 1363 vw, 1344 w, 1308 w, 1281 vw, 1255 w, 1227 m, 1146 m, 764 s, 550 s, 459 m cm−1. 1H-NMR (600 MHz, CDCl3) δ: 7.50 (s, br, 2H, NH), 3.27 (q, 4H, CH2CH2CH3), 1.58 (q, 4H, CH2CH2CH3), 0.95 (t, 6H, CH2CH2CH3) ppm. 13C{1H}-NMR (151 MHz, CDCl3) δ: 160.1, 41.5, 22.7, 11.4 ppm.
4. Conclusions
N,N′-Dipropyloxamide (1) was synthesised starting from diethyloxalate with n-propylamine and structurally characterised by single crystal X-ray diffraction.
The crystal structure of compound 1 shows the antiperiplanar conformation peculiar to planar oxalamide core with the n-propyl alkyl chains protruding on opposite sides.
In the crystal packing, several intermolecular interactions can be observed, the main ones consisting of N–H···O HBs between adjacent molecules featuring oxalamides core lying on the same plane.
These HB interactions are typical of N,N′-dialkyloxamides, and the resulting self-assembly behaviour can have applications in different fields of crystal engineering.
Supplementary Materials
The following supporting information can be downloaded online. Figure S1: FT-IR spectrum; Figure S2: 1H-NMR spectrum; Figure S3: 13C{1H}-NMR spectrum; Table S1: crystal data and refinement parameters; Table S2: bond Lengths; Table S3: bond Angles; Table S4: torsion Angles.
Author Contributions
Conceptualisation: A.P.; Methodology: A.P., M.A. and M.C.A.; Investigation: A.P., E.D. and E.P.; Data Curation: A.P., E.D., E.P. and S.J.C.; Writing—original draft preparation: A.P.; Writing—review and editing: A.P., E.D., E.P., M.A., M.C.A., V.L. and S.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge Fondazione di Sardegna (FdS Progetti Biennali di Ateneo, annualità 2020) for financial support.
Data Availability Statement
Crystallographic data were deposited at CCCD (CIF deposition number 2310519).
Acknowledgments
CeSAR (Centro Servizi di Ateneo per la Ricerca) of the University of Cagliari is kindly acknowledged for NMR facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Qiao, T.; Xiong, Y.; Feng, Y.; Guo, W.; Zhou, Y.; Zhao, J.; Jiang, T.; Shi, C.; Han, Y. Inhibition of LDH-A by Oxamate Enhances the Efficacy of Anti-PD-1 Treatment in an NSCLC Humanized Mouse Model. Front. Oncol. 2021, 11, 1033. [Google Scholar] [CrossRef] [PubMed]
- Miskimins, W.K.; Ahn, H.J.; Kim, J.Y.; Ryu, S.; Jung, Y.-S. Synergistic Anti-Cancer Effect of Phenformin and Oxamate. PLoS ONE 2014, 9, 85576. [Google Scholar] [CrossRef] [PubMed]
- Ogbu, I.M.; Kurtay, G.; Robert, F.; Landais, Y. Oxamic Acids: Useful Precursors of Carbamoyl Radicals. Chem. Commun. 2022, 58, 7593–7607. [Google Scholar] [CrossRef] [PubMed]
- Ogbu, I.M.; Lusseau, J.; Kurtay, G.; Robert, F.; Landais, Y. Urethanes Synthesis from Oxamic Acids under Electrochemical Conditions. Chem. Commun. 2020, 56, 12226–12229. [Google Scholar] [CrossRef] [PubMed]
- Maiore, L.; Aragoni, M.C.; Carcangiu, G.; Cocco, O.; Isaia, F.; Lippolis, V.; Meloni, P.; Murru, A.; Slawin, A.M.Z.; Tuveri, E.; et al. Oxamate Salts as Novel Agents for the Restoration of Marble and Limestone Substrates: Case Study of Ammonium N-Phenyloxamate. New J. Chem. 2016, 40, 2768–2774. [Google Scholar] [CrossRef]
- Pintus, A.; Aragoni, M.C.; Carcangiu, G.; Giacopetti, L.; Isaia, F.; Lippolis, V.; Maiore, L.; Meloni, P.; Arca, M. Density Functional Theory Modelling of Protective Agents for Carbonate Stones: A Case Study of Oxalate and Oxamate Inorganic Salts. New J. Chem. 2018, 42, 11593–11600. [Google Scholar] [CrossRef]
- Maiore, L.; Aragoni, M.C.; Carcangiu, G.; Cocco, O.; Isaia, F.; Lippolis, V.; Meloni, P.; Murru, A.; Tuveri, E.; Arca, M. Synthesis, Characterization and DFT-Modeling of Novel Agents for the Protection and Restoration of Historical Calcareous Stone Substrates. J. Colloid Interface Sci. 2015, 448, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Aragoni, M.C.; Giacopetti, L.; Arca, M.; Carcangiu, G.; Columbu, S.; Gimeno, D.; Isaia, F.; Lippolis, V.; Meloni, P.; Ezquerra, A.N.; et al. Ammonium Monoethyloxalate (AmEtOx): A New Agent for the Conservation of Carbonate Stone Substrates. New J. Chem. 2021, 45, 5327–5339. [Google Scholar] [CrossRef]
- Pintus, A.; Aragoni, M.C.; Carcangiu, G.; Caria, V.; Coles, S.J.; Dodd, E.; Giacopetti, L.; Gimeno, D.; Lippolis, V.; Meloni, P.; et al. Ammonium N-(Pyridin-2-Ylmethyl)Oxamate (AmPicOxam): A Novel Precursor of Calcium Oxalate Coating for Carbonate Stone Substrates. Molecules 2023, 28, 5768. [Google Scholar] [CrossRef]
- Curreli, F.; Kwon, Y.D.; Zhang, H.; Scacalossi, D.; Belov, D.S.; Tikhonov, A.A.; Andreev, I.A.; Altieri, A.; Kurkin, A.V.; Kwong, P.D.; et al. Structure-Based Design of a Small Molecule CD4-Antagonist with Broad Spectrum Anti-HIV-1 Activity. J. Med. Chem. 2015, 58, 6909–6927. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Agnelli, G. Edoxaban: A Focused Review of Its Clinical Pharmacology. Eur. Heart J. 2014, 35, 1844–1855. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Elangovan, S.; Sang, R.; Spannenberg, A.; Jackstell, R.; Junge, K.; Li, Y.; Beller, M. Selective Catalytic Two-Step Process for Ethylene Glycol from Carbon Monoxide. Nat. Commun. 2016, 7, 12075. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.Q.; Zhou, Q.Q.; Diskin-Posner, Y.; Ben-David, Y.; Milstein, D. Synthesis of Oxalamides by Acceptorless Dehydrogenative Coupling of Ethylene Glycol and Amines and the Reverse Hydrogenation Catalyzed by Ruthenium. Chem. Sci. 2020, 11, 7188–7193. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, Y.; Zhang, L.; Guo, Y.; Ma, D. Oxalic Diamides and Tert-Butoxide: Two Types of Ligands Enabling Practical Access to Alkyl Aryl Ethers via Cu-Catalyzed Coupling Reaction. J. Am. Chem. Soc. 2019, 141, 3541–3549. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Frank, W.; Reiss, G.J.; Ganter, C. An N-Heterocyclic Carbene Ligand with an Oxalamide Backbone. Organometallics 2010, 29, 4418–4420. [Google Scholar] [CrossRef]
- Alemán, C.; Casanovas, J. Analysis of the Oxalamide Functionality as Hydrogen Bonding Former: Geometry, Energetics, Cooperative Effects, NMR Chemical Characterization and Implications in Molecular Engineering. J. Mol. Struct. THEOCHEM 2004, 675, 9–17. [Google Scholar] [CrossRef]
- Coe, S.; Kane, J.J.; Nguyen, T.L.; Toledo, L.M.; Wininger, E.; Fowler, F.W.; Lauher, J.W. Molecular Symmetry and the Design of Molecular Solids: The Oxalamide Functionality as a Persistent Hydrogen Bonding Unit. J. Am. Chem. Soc. 1997, 119, 86–93. [Google Scholar] [CrossRef]
- Lauher, J.W.; Fowler, F.W.; Goroff, N.S. Single-Crystal-to-Single-Crystal Topochemical Polymerizations by Design. Acc. Chem. Res. 2008, 41, 1215–1229. [Google Scholar] [CrossRef]
- Curtis, S.M.; Le, N.; Fowler, F.W.; Lauher, J.W. A Rational Approach to the Preparation of Polydipyridyldiacetylenes: An Exercise in Crystal Design. Cryst. Growth Des. 2005, 5, 2313–2321. [Google Scholar] [CrossRef]
- Nowick, J.S. Exploring β-Sheet Structure and Interactions with Chemical Model Systems. Acc. Chem. Res. 2008, 41, 1319–1330. [Google Scholar] [CrossRef]
- Frkanec, L.; Žinić, M. Chiral Bis(Amino Acid)- and Bis(Amino Alcohol)-Oxalamide Gelators. Gelation Properties, Self-Assembly Motifs and Chirality Effects. Chem. Commun. 2010, 46, 522–537. [Google Scholar] [CrossRef]
- Makarević, J.; Jokić, M.; Frkanec, L.; Katalenić, D.; Žinić, M. Gels with Exceptional Thermal Stability Formed by Bis(Amino Acid) Oxalamide Gelators and Solvents of Low Polarity. Chem. Commun. 2002, 2238–2239. [Google Scholar] [CrossRef]
- Asn, L.; Armelin, E.; Montan, J.; Rodrguez-Galn, A.; Puiggal, J. Sequential Poly(Ester Amide)s Based on Glycine, Diols, and Dicarboxylic Acids: Thermal Polyesterification versus Interfacial Polyamidation. Characterization of Polymers Containing Stiff Units. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 4283–4293. [Google Scholar] [CrossRef]
- Sun, D.L.; Ye, J.H.; Fang, Y.X.; Chao, Z.S. Green Synthesis of N,N′-Dialkylureas from CO2 and Amines Using Metal Salts of Oxalates as Catalysts. Ind. Eng. Chem. Res. 2016, 55, 64–70. [Google Scholar] [CrossRef]
- Zharkov, M.N.; Kuchurov, I.V.; Fomenkov, I.V.; Tartakovsky, V.A.; Fedyanin, I.V.; Zlotin, S.G. Safe and Convenient Synthesis of Primary N-Nitramines in the Freon Media. Synthesis 2017, 49, 1103–1108. [Google Scholar] [CrossRef]
- Larson, D.B.; McGlynn, S.P. The Electronic Spectroscopy of Oxamides. J. Mol. Spectrosc. 1973, 47, 469–490. [Google Scholar] [CrossRef]
- Meeks, J.L.; McGlynn, S.P. Photoelectron Spectra of Carbonyls. Oxamide, Parabanic Acid, and Their N-Methyl Derivatives. J. Am. Chem. Soc. 1975, 97, 5079–5083. [Google Scholar] [CrossRef]
- Desseyn, H.O.; Perlepes, S.P.; Clou, K.; Blaton, N.; Van Der Veken, B.J.; Dommisse, R.; Hansen, P.E. Theoretical, Structural, Vibrational, NMR, and Thermal Evidence of the Inter- versus Intramolecular Hydrogen Bonding in Oxamides and Thiooxamides. J. Phys. Chem. A 2004, 108, 5175–5182. [Google Scholar] [CrossRef]
- Santana, M.D.; García, G.; Julve, M.; Lloret, F.; Pérez, J.; Liu, M.; Sanz, F.; Cano, J.; López, G. Oxamidate-Bridged Dinuclear Five-Coordinate Nickel(II) Complexes: A Magneto−Structural Study. Inorg. Chem. 2004, 43, 2132–2140. [Google Scholar] [CrossRef]
- Casellato, U.; Guerriero, P.; Tamburini, S.; Vigato, P.A. Metal Complexes with Disubstituted Oxamidic Ligands. Inorganica Chim. Acta 1997, 260, 1–9. [Google Scholar] [CrossRef]
- Meyer, T.; Rabeah, J.; Brückner, A.; Wu, X.F. Visible-Light-Induced Palladium-Catalyzed Dehydrogenative Carbonylation of Amines to Oxalamides. Chem. A Eur. J. 2021, 27, 5642–5647. [Google Scholar] [CrossRef]
- Rice, L.M.; Grogan, C.H.; Emmet, R. N,N′-Dialkyloxamides. J. Am. Chem. Soc. 1953, 75, 242. [Google Scholar] [CrossRef]
- CCDC Searches Were Performed Using ConQuest Version 2022.1.0.
- Etter, M.C. Encoding and Decoding Hydrogen-Bond Patterns of Organic Compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chemie Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Scott, A.; Dinkelmeyer, B.; Fowler, F.W.; Lauher, J.W. Design of Molecular Solids: Utility of the Hydroxyl Functionality as a Predictable Design Element. New J. Chem. 1998, 22, 129–135. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Podda, E.; Caria, V.; Carta, S.A.; Cherchi, M.F.; Lippolis, V.; Murgia, S.; Orrù, G.; Pippia, G.; Scano, A.; et al. [AuIII(N^N)Br2](PF6): A Class of Antibacterial and Antibiofilm Complexes (N^N = 2,2′-Bipyridine and 1,10-Phenanthroline Derivatives). Inorg. Chem. 2022, 62, 2924–2933. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.H.; Li, X.M.; Wang, L.; Zhang, S.S. N,N′-Diphenyloxalamide. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, o2185–o2186. [Google Scholar] [CrossRef]
- Wen, Y.H.; Xu, L.L.; Li, X.M.; Zhang, S.S. N,N′-Bis(2-Methylphenyl)Oxamide. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, o3276–o3277. [Google Scholar] [CrossRef]
- Wen, Y.H.; Zhang, K.; Li, X.M.; Bi, S.; Zhang, S.S. N,N′-Bis(3-Methoxyphenyl)Oxamide. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, o3443–o3444. [Google Scholar] [CrossRef]
- Podda, E.; Dodd, E.; Arca, M.; Aragoni, M.C.; Lippolis, V.; Coles, S.J.; Pintus, A. N,N′-Dibutyloxamide. Molbank 2023, 2023, M1677. [Google Scholar] [CrossRef]
- Sheldrick, G.M. IUCr SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Huisgen, R.; Laschtuvka, E. Eine Neue Synthese von Derivaten Des Pyrrols. Chem. Ber. 1960, 93, 65–81. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).