4,4′-([2,2′-Bithiophene]-5,5′-diylbis(ethyne-2,1-diyl))bis(1-methylpyridin-1-ium) Iodide
Abstract
:1. Introduction
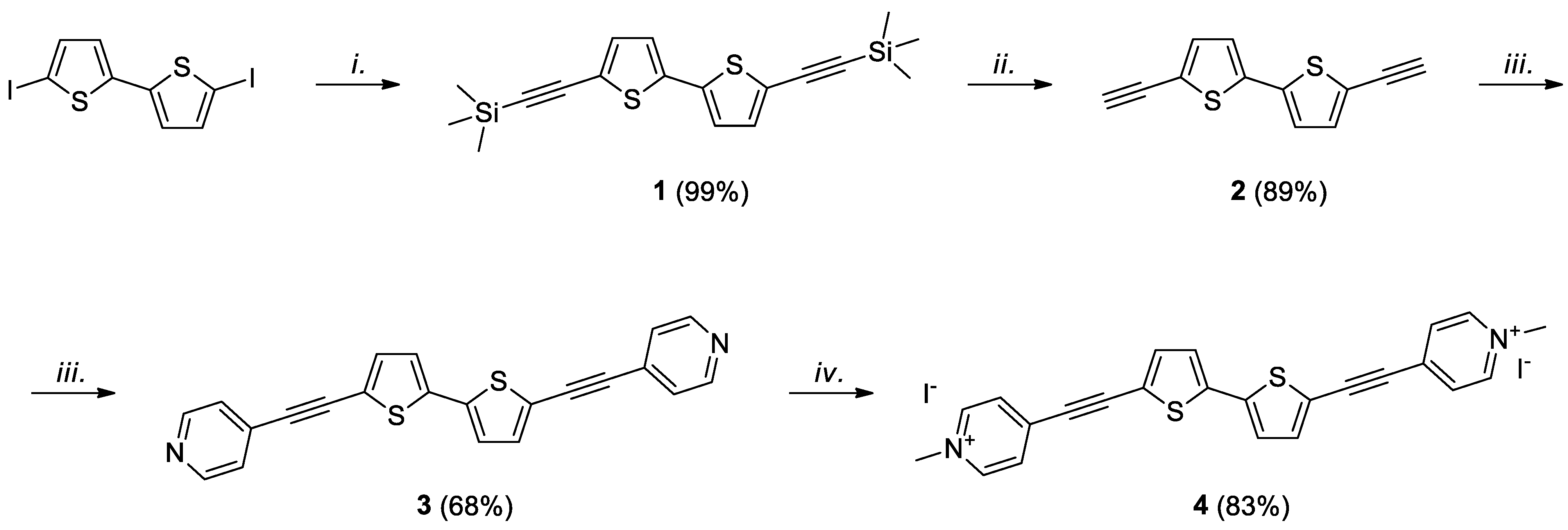
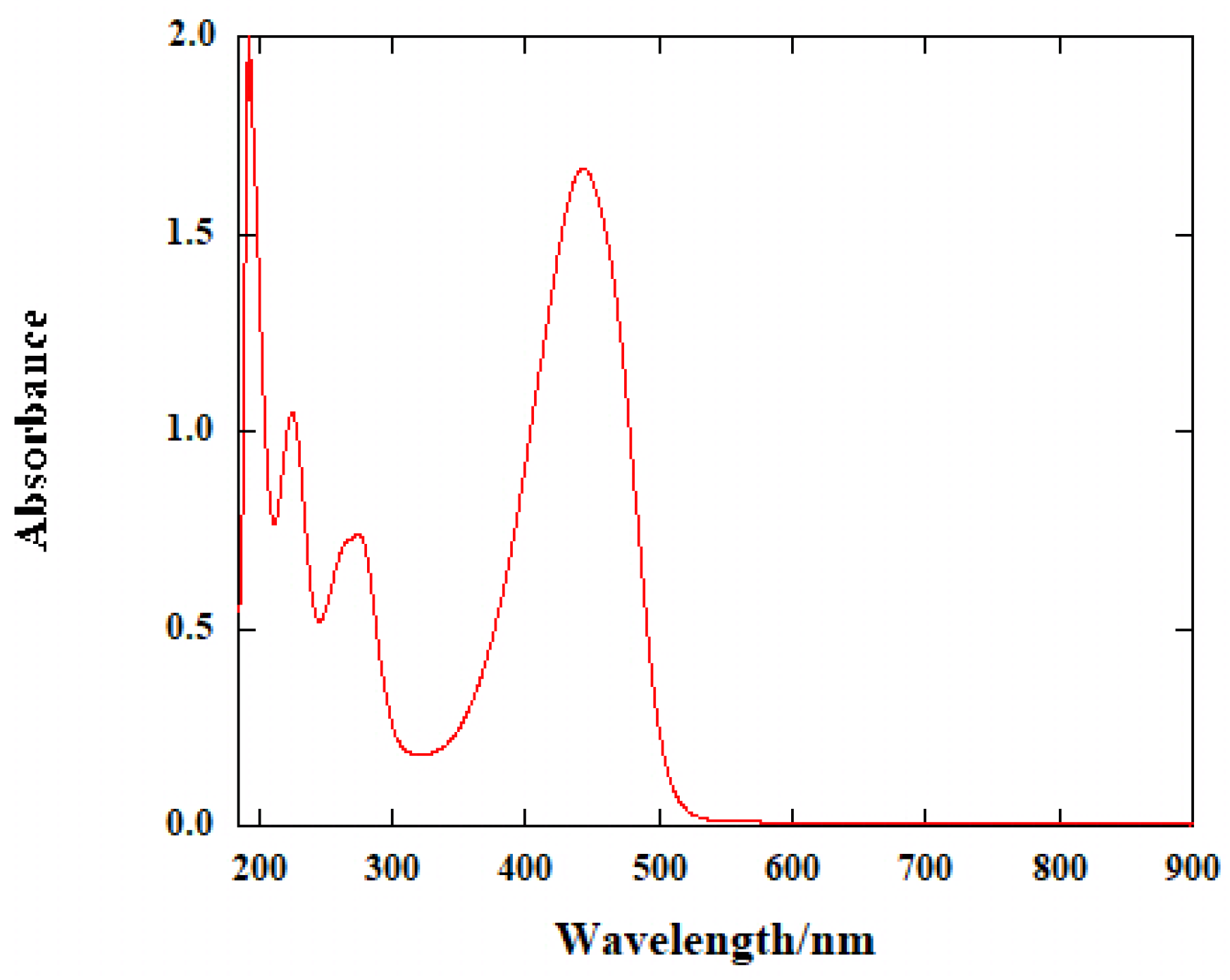
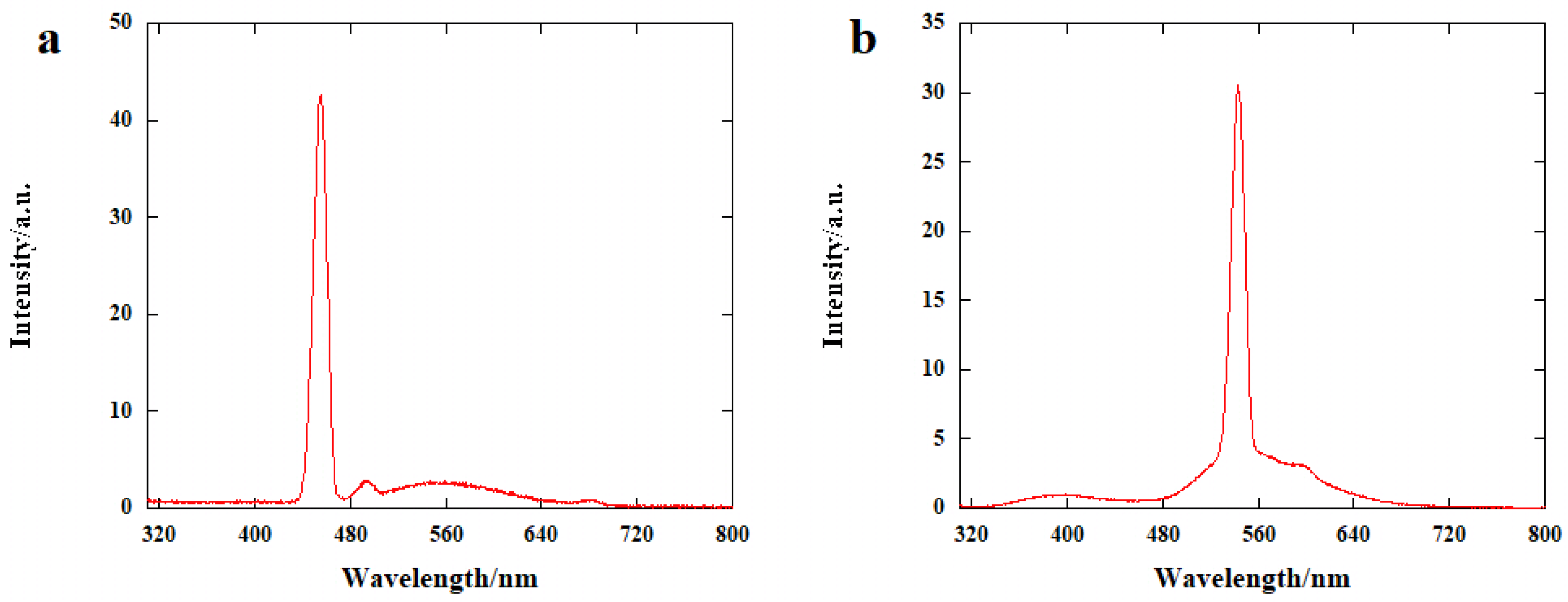
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Di Maria, F.; Zangoli, M.; Barbarella, G. Supramolecular Thiophene-Based Materials: A Few Examples of the Interplay between Synthesis, Optoelectronic Properties and Applications. Org. Mater. 2021, 3, 321–336. [Google Scholar] [CrossRef]
- Caballero, R.; Cohen, B.; Gutiérrez, M. Thiophene-Based Covalent Organic Frameworks: Synthesis, Photophysics and Light-Driven Applications. Molecules 2021, 26, 7666. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Ma, C.Q.; Bäuerle, P. Functional Oligothiophenes: Molecular Design for Multidimensional Nanoarchitectures and Their Applications. Chem. Rev. 2009, 109, 1141–1276. [Google Scholar] [CrossRef] [PubMed]
- Bobade, R.S. Polythiophene composites: A review of selected applications. J. Polym. Eng. 2011, 31, 209–215. [Google Scholar] [CrossRef]
- Xue, Z.; Chen, S.; Gao, N.; Xue, Y.; Lu, B.; Watson, O.A.; Zang, L.; Xu, J. Structural Design and Applications of Stereoregular Fused Thiophenes and Their Oligomers and Polymers. Polym. Rev. 2020, 60, 318–358. [Google Scholar]
- Zangoli, M.; Di Maria, F. Synthesis, characterization, and biological applications of semiconducting polythiophene-based nanoparticles. View 2021, 2, 20200086. [Google Scholar] [CrossRef]
- Turkoglu, G.; Cinar, M.E.; Ozturk, T. Thiophene-based organic semiconductors. Top. Curr. Chem. (Z) 2017, 375, 84. [Google Scholar] [CrossRef]
- Kaloni, T.P.; Giesbrecht, P.K.; Schreckenbach, G.; Freund, M.S. Polythiophene: From Fundamental Perspectives to Applications. Chem. Mater. 2017, 29, 10248–10283. [Google Scholar] [CrossRef]
- Rasmussen, S.C.; Evenson, S.J.; McCausland, C.B. Fluorescent thiophene-based materials and their outlook for emissive applications. Chem. Commun. 2015, 51, 4528–4543. [Google Scholar]
- Larik, F.A.; Faisal, M.; Saeed, A.; Abbas, Q.; Kazi, M.A.; Abbas, N.; Thebo, A.A.; Khan, D.M.; Channar, P.A. Thiophene-based molecular and polymeric semiconductors for organic field effect transistors and organic thin film transistors. J. Mater. Sci. Mater. Electron. 2018, 29, 17975–18010. [Google Scholar] [CrossRef]
- Idrissi, A.; El Fakir, Z.; Atir, R.; Habsaoui, A.; Touhami, M.E.; Bouzakraoui, S. Thiophene-based molecules as hole transport materials for efficient perovskite solar cells or as donors for organic solar cells. Mater. Chem. Phys. 2023, 293, 126851. [Google Scholar]
- Balasaravanan, R.; Kuan, C.-H.; Hsu, S.-M.; Chang, E.-C.; Chen, Y.-C.; Tsai, Y.-T.; Jhou, M.-L.; Yau, S.-L.; Liu, C.-L.; Chen, M.-C.; et al. Triphenylamine (TPA)-Functionalized Structural Isomeric Polythiophenes as Dopant Free Hole-Transporting Materials for Tin Perovskite Solar Cells . Adv. Energy Mater. 2023, 2302047. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Shetty, N.S.; Mahesha, P.; Gaonkar, S.L. A Comprehensive Review on Thiophene Based Chemosensors. J. Fluoresc. 2022, 32, 19–56. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, R.-M.; Camurlu, P. Review—Functional Platforms for (Bio)sensing: Thiophene-Pyrrole Hybrid Polymers. J. Electrochem. Soc. 2020, 167, 037557. [Google Scholar] [CrossRef]
- Striepe, L.; Baumgartner, T. Viologens and Their Application as Functional Materials. Chem. Eur.J. 2017, 23, 16924–16940. [Google Scholar] [CrossRef]
- Kathiresan, M.; Ambrose, B.; Angulakshmi, N.; Mathew, D.E.; Sujatha, D.; Stephan, A.M. Viologens: A versatile organic molecule for energy storage applications. J. Mater. Chem. A 2021, 9, 27215–27233. [Google Scholar] [CrossRef]
- Ding, J.; Zheng, C.; Wang, L.; Lu, C.; Zhang, B.; Chen, Y.; Li, M.; Zhai, G.; Zhuang, X. Viologen-inspired functional materials: Synthetic strategies and applications. J. Mater. Chem. A 2019, 7, 23337–23360. [Google Scholar] [CrossRef]
- Arundhathi, K.V.; Vaishnavi, P.; Aneeja, T.; Anilkumar, G. Copper-catalyzed Sonogashira reactions: Advances and perspectives since 2014. RSC Adv. 2023, 13, 4823–4834. [Google Scholar] [CrossRef]
- Bryce, M.R. A review of functional linear carbon chains (oligoynes, polyynes, cumulenes) and their applications as molecular wires in molecular electronics and optoelectronics. J. Mater. Chem. C 2021, 9, 10524–10546. [Google Scholar] [CrossRef]
- Seri, M.; Marrocchi, A. The carbon–carbon triple bond as a tool to design organic semiconductors for photovoltaic applications: An assessment of prospects and challenges. J. Mater. Chem. C 2021, 9, 16164–16186. [Google Scholar] [CrossRef]
- Neenan, T.X.; Whitesides, G.M. Synthesis of high carbon materials from acetylenic precursors. Preparation of aromatic monomers bearing multiple ethynyl groups. J. Org. Chem. 1988, 53, 2489–2496. [Google Scholar]
- Hwang, E.; Lusker, K.L.; Garno, J.C.; Losovyj, Y.; Nesterov, E.E. Semiconducting polymer thin films by surface-confined stepwise click polymerization. Chem. Commun. 2011, 47, 11990–11992. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romagnoli, L.; D’Annibale, A.; Latini, A. 4,4′-([2,2′-Bithiophene]-5,5′-diylbis(ethyne-2,1-diyl))bis(1-methylpyridin-1-ium) Iodide. Molbank 2023, 2023, M1733. https://doi.org/10.3390/M1733
Romagnoli L, D’Annibale A, Latini A. 4,4′-([2,2′-Bithiophene]-5,5′-diylbis(ethyne-2,1-diyl))bis(1-methylpyridin-1-ium) Iodide. Molbank. 2023; 2023(4):M1733. https://doi.org/10.3390/M1733
Chicago/Turabian StyleRomagnoli, Lorenza, Andrea D’Annibale, and Alessandro Latini. 2023. "4,4′-([2,2′-Bithiophene]-5,5′-diylbis(ethyne-2,1-diyl))bis(1-methylpyridin-1-ium) Iodide" Molbank 2023, no. 4: M1733. https://doi.org/10.3390/M1733
APA StyleRomagnoli, L., D’Annibale, A., & Latini, A. (2023). 4,4′-([2,2′-Bithiophene]-5,5′-diylbis(ethyne-2,1-diyl))bis(1-methylpyridin-1-ium) Iodide. Molbank, 2023(4), M1733. https://doi.org/10.3390/M1733






