(2aR,2a1S,5aR,9bR)-4-Isopropyl-7,8-dimethoxy-2a1-methyl-2,2a,2a1,3,5a,9b-hexahydrofluoreno[9,1-bc]furan
Abstract
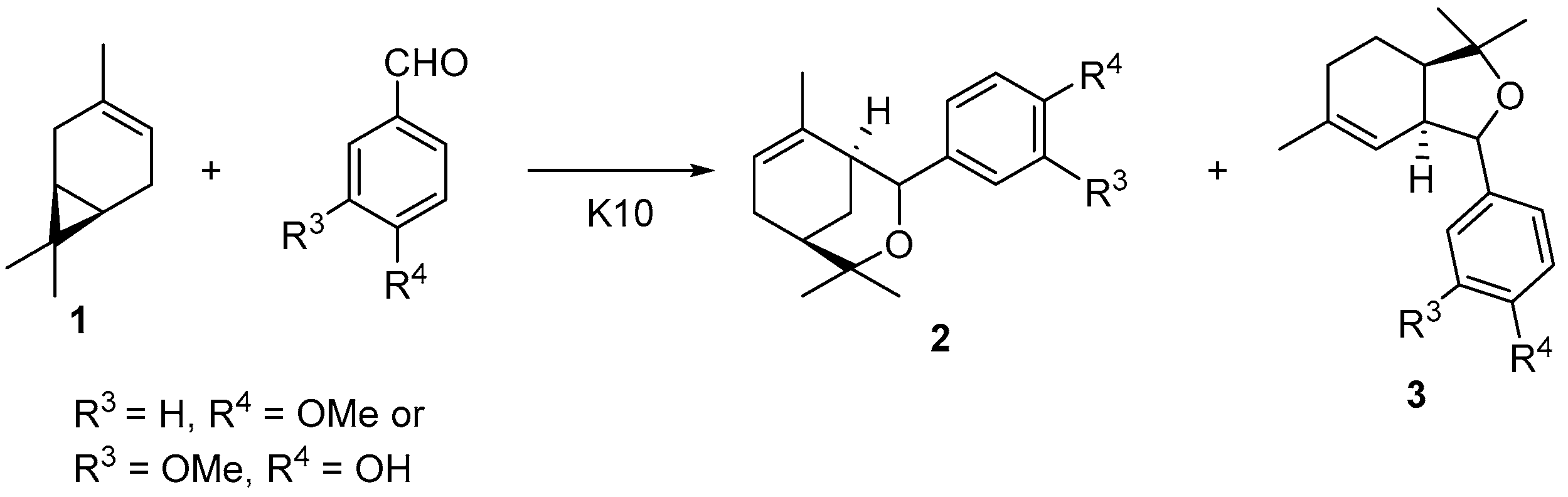
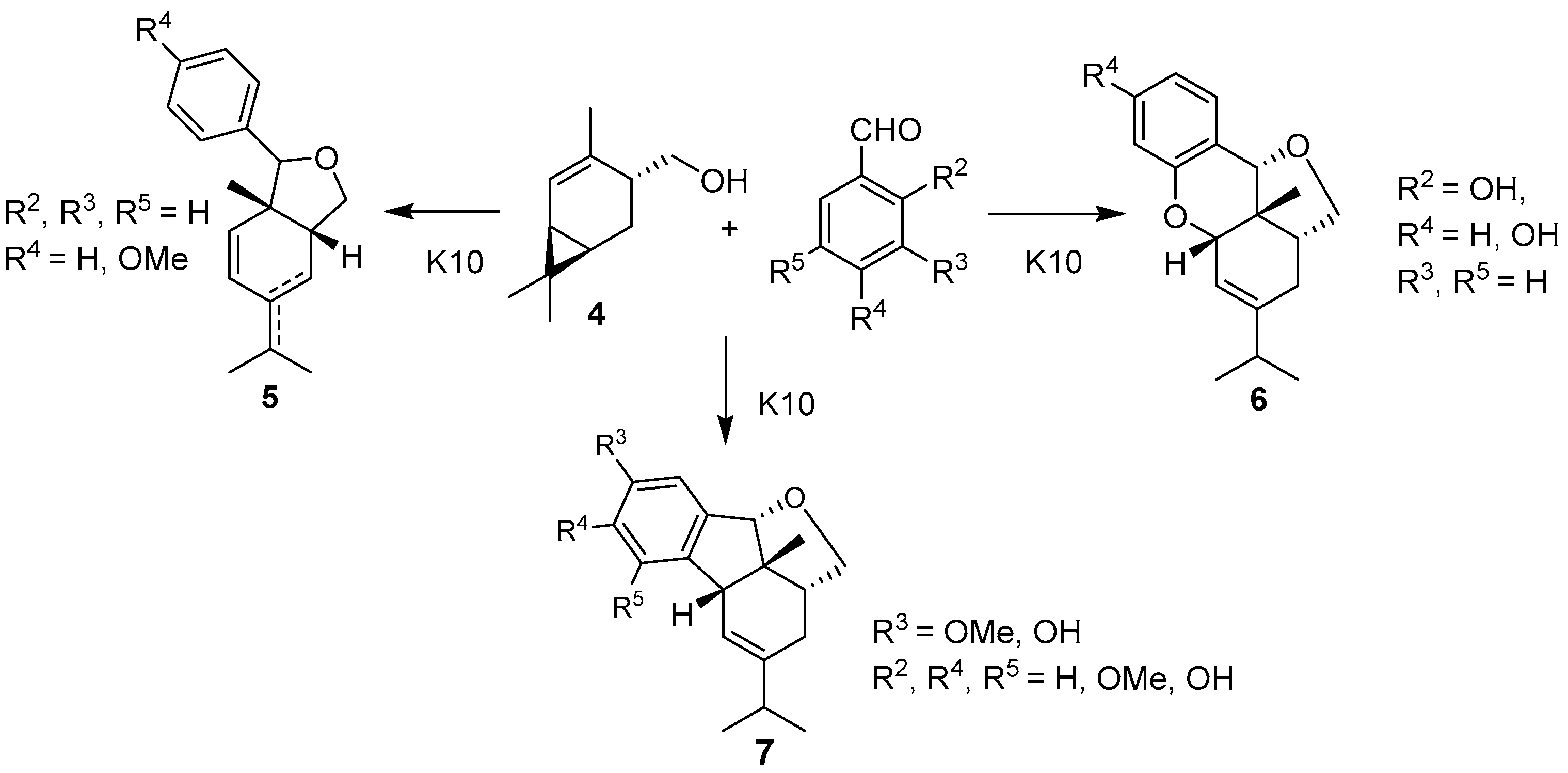
:1. Introduction
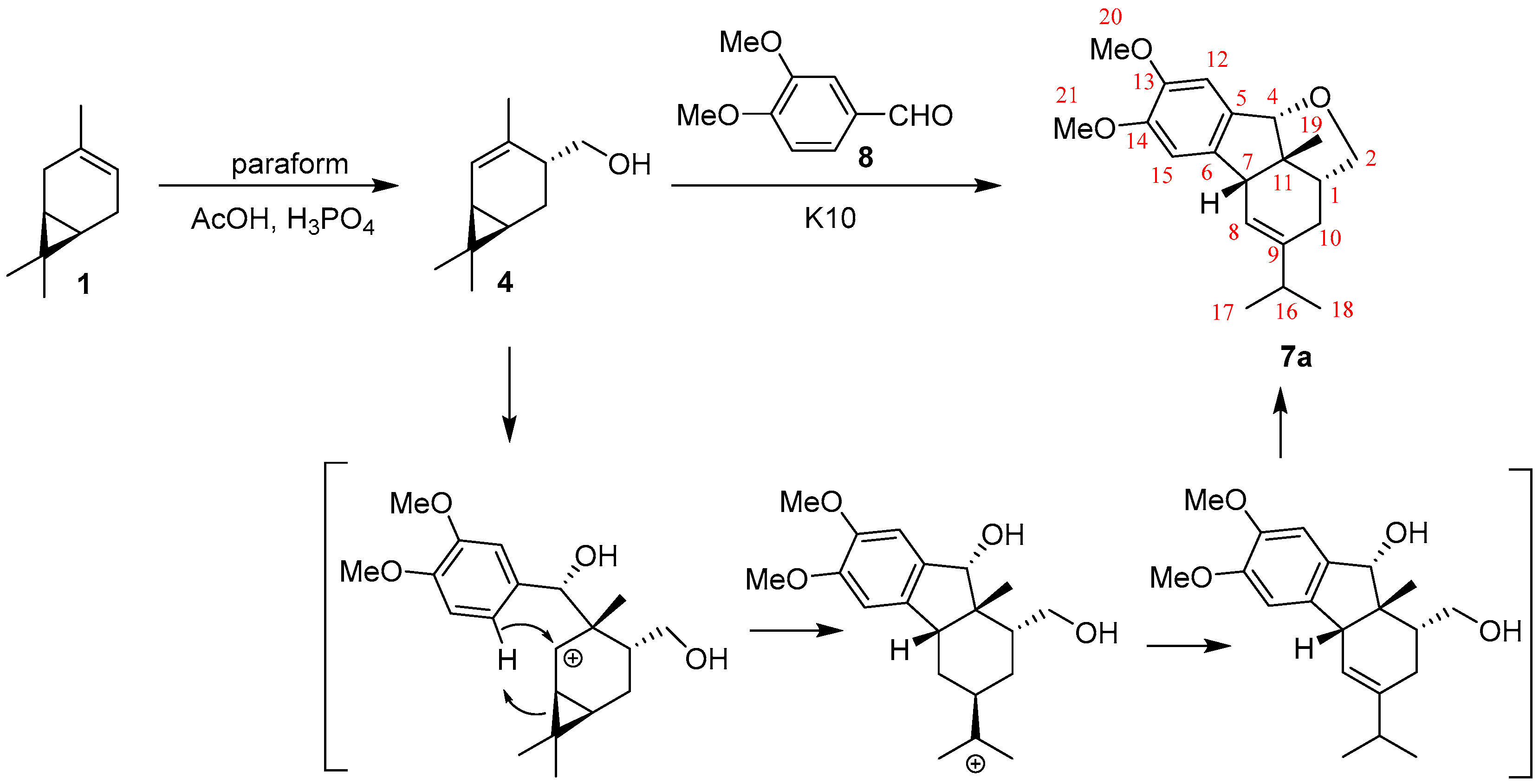
2. Results
3. Materials and Methods
3.1. General
3.2. Synthesis of (2aR,2a1S,5aR,9bR)-4-Isopropyl-7,8-dimethoxy-2a1-methyl-2,2a,2a1,3,5a,9b-hexahydrofluoreno[9,1-bc]furan 7a
3.3. X-ray Diffraction Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives—Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef]
- Salakhutdinov, N.F.; Volcho, K.P.; Yarovaya, O.I. Monoterpenes as a renewable source of biologically active compounds. Pure Appl. Chem. 2017, 89, 1105–1117. [Google Scholar] [CrossRef]
- Patrusheva, O.S.; Volcho, K.P.; Salakhutdinov, N.F. Approaches to the synthesis of oxygen-containing heterocyclic compounds based on monoterpenoids. Russ. Chem. Rev. 2018, 87, 771–796. [Google Scholar] [CrossRef]
- Newman, D.J. Natural products as leads to potential drugs: An old process or the new hope for drug discovery? J. Med. Chem. 2008, 51, 2589–2599. [Google Scholar] [CrossRef]
- Il’ina, I.V.; Volcho, K.P.; Korchagina, D.V.; Salnikov, G.E.; Genaev, A.M.; Karpova, E.V.; Salakhutdinov, N.F. Unusual Reactions of (+)-Car-2-ene and (+)-Car-3-ene with Aldehydes on K10 Clay. Helv. Chim. Acta 2010, 93, 2135–2150. [Google Scholar] [CrossRef]
- Il’ina, I.V.; Dyrkheeva, N.S.; Zakharenko, A.L.; Sidorenko, A.Y.; Li-Zhulanov, N.S.; Korchagina, D.V.; Chand, R.; Ayine-Tora, D.M.; Chepanova, A.A.; Zakharova, O.D.; et al. Design, synthesis, and biological investigation of novel classes of 3-carene-derived potent inhibitors of TDP1. Molecules 2020, 25, 3496. [Google Scholar] [CrossRef]
- Saudagar, P.; Saha, P.; Saikia, A.K.; Dubey, V.K. Molecular mechanism underlying antileishmanial effect of oxabicyclo[3.3.1]nonanones: Inhibition of key redox enzymes of the pathogen. Eur. J. Pharm. Biopharm. 2013, 85, 569. [Google Scholar] [CrossRef]
- Prakash, S.; Shyam, L.M.; Pipas, S.; Anil, K.S.; Shyam, S.; Vikash, K.D. In Vivo Assessment of Antileishmanial Property of 4-(4,4,8-Trimethyl-7- oxo-3-oxabicyclo[3.3.1]non-2-yl)-benzoic Acid Methyl Ester, an Oxabicyclo[ 3.3.1]nonanones. Lett. Drug Des. Discov. 2014, 117, 937–938. [Google Scholar] [CrossRef]
- Hamann, L.G.; Meyer, J.H.; Ruppar, D.A.; Marschke, K.B.; Lopez, F.J.; Allegretto, E.A.; Karanewsky, D.S. Structure–activity relationships and sub-type selectivity in an oxabicyclic estrogen receptor α/β agonist scaffold. Bioorg. Med. Chem. Lett. 2005, 15, 1463–1466. [Google Scholar] [CrossRef]
- Nakamura, M.; Niiyama, K.; Yamakawa, T. Versatile method for the synthesis of 4-substituted 6-methyl-3-oxabicyclo[3.3.1]non-6-ene-1-methanol derivatives: Prins-type cyclization reaction catalyzed by hafnium triflate. Tetrahedron Lett. 2009, 50, 6462–6465. [Google Scholar] [CrossRef]
- Sibley, R.; Hatoum-Mokdad, H.; Schoenleber, R.; Musza, L.; Stirtan, W.; Marrero, D.; Carley, W.; Xiao, H.; Dumas, J. A novel estrogen receptor ligand template. Bioorg. Med. Chem. Lett. 2003, 13, 1919–1922. [Google Scholar] [CrossRef]
- Hsieh, R.W.; Rajan, S.S.; Sharma, S.K.; Guo, Y.; De Sombre, E.R.; Mrksich, M.; Greene, G.L.J. Identification of Ligands with Bicyclic Scaffolds Provides Insights into Mechanisms of Estrogen Receptor Subtype Selectivity. Biol. Chem. 2006, 281, 17909–17919. [Google Scholar] [CrossRef] [PubMed]
- Ohloff, G.; Farnow, H.; Philipp, W. Zur Kenntnis homologer Alkohole der Terpen- und Sesquiterpenreihe X. Synthese Des (+)-3-Hydroxymethyl-Δ4-Carens. Justus Liebigs Ann. Chem. 1958, 613, 43–55. [Google Scholar] [CrossRef]
- Sidorenko, A.Y.; Kurban, Y.M.; Kravtsova, A.V.; Il’ina, I.V.; Li-Zhulanov, N.S.; Korchagina, D.V.; Sanchez-Velandia, J.E.; Aho, A.; Volcho, K.P.; Salakhutdinov, N.F.; et al. Clays catalyzed cascade Prins and Prins-Friedel-Crafts reactions for synthesis of terpenoid-derived polycyclic compounds. Appl. Catal. 2022, 629, 118395. [Google Scholar] [CrossRef]
- Kurbakova, S.Y.; Il‘ina, I.V.; Mikhalchenko, O.S.; Pokrovsky, M.A.; Korchagina, D.V.; Volcho, K.P.; Pokrovsky, A.G.; Salakhutdinov, N.F. The short way to chiral compounds with hexahydrofluoreno[9,1-bc]furan framework: Synthesis and cytotoxic activity. Bioorg. Med. Chem. 2015, 23, 1472–1480. [Google Scholar] [CrossRef]
- Sakata, Y.; Yasui, E.; Takatori, K.; Suzuki, Y.; Mizukami, M.; Nagumo, S. Syntheses of Polycyclic Tetrahydrofurans by Cascade Reactions Consisting of Five-Membered Ring Selective Prins Cyclization and Friedel–Crafts Cyclization. J. Org. Chem. 2018, 83, 9103–9118. [Google Scholar] [CrossRef]
- Roy, S. Prins-Friedel-Crafts cyclization: Synthesis of diversely functionalized six-membered oxacycles. Curr. Org. Chem. 2021, 25, 635–651. [Google Scholar] [CrossRef]
- Padmaja, P.; Narayana Reddy, P.; Subba Reddy, B.V. Tandem Prins cyclizations for the construction of oxygen containing heterocycles. Org. Biomol. Chem. 2020, 18, 7514–7532. [Google Scholar] [CrossRef]
- Lv, L.; Lu, S.; Chen, Y.; Li, Z. Diastereoselective building up polycyclic tetrahydrofurans via tandem annulation of 1,n-enynes with aliphatic acids. Org. Chem. Front. 2017, 4, 2147–2152. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, H.; Zhu, Y.; Zhou, T.; He, J.; Chen, N.; Lang, M.; Li, H.; Wang, J. Copper-Catalyzed Chemo- and Diastereoselective 1,3-Dipolar Cycloaddition of Carbonyl Ylide and Aldehyde-Tethered-Cyclohexadienone to Access Polycyclic Systems. Adv. Synth. Catal. 2021, 363, 4532–4537. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyina, I.V.; Li-Zhulanov, N.S.; Gatilov, Y.V.; Volcho, K.P.; Sidorenko, A.Y.; Agabekov, V.E.; Salakhutdinov, N.F. (2aR,2a1S,5aR,9bR)-4-Isopropyl-7,8-dimethoxy-2a1-methyl-2,2a,2a1,3,5a,9b-hexahydrofluoreno[9,1-bc]furan. Molbank 2023, 2023, M1734. https://doi.org/10.3390/M1734
Ilyina IV, Li-Zhulanov NS, Gatilov YV, Volcho KP, Sidorenko AY, Agabekov VE, Salakhutdinov NF. (2aR,2a1S,5aR,9bR)-4-Isopropyl-7,8-dimethoxy-2a1-methyl-2,2a,2a1,3,5a,9b-hexahydrofluoreno[9,1-bc]furan. Molbank. 2023; 2023(4):M1734. https://doi.org/10.3390/M1734
Chicago/Turabian StyleIlyina, Irina V., Nikolai S. Li-Zhulanov, Yuri V. Gatilov, Konstantin P. Volcho, Alexander Yu. Sidorenko, Vladimir E. Agabekov, and Nariman F. Salakhutdinov. 2023. "(2aR,2a1S,5aR,9bR)-4-Isopropyl-7,8-dimethoxy-2a1-methyl-2,2a,2a1,3,5a,9b-hexahydrofluoreno[9,1-bc]furan" Molbank 2023, no. 4: M1734. https://doi.org/10.3390/M1734
APA StyleIlyina, I. V., Li-Zhulanov, N. S., Gatilov, Y. V., Volcho, K. P., Sidorenko, A. Y., Agabekov, V. E., & Salakhutdinov, N. F. (2023). (2aR,2a1S,5aR,9bR)-4-Isopropyl-7,8-dimethoxy-2a1-methyl-2,2a,2a1,3,5a,9b-hexahydrofluoreno[9,1-bc]furan. Molbank, 2023(4), M1734. https://doi.org/10.3390/M1734





