trans-3-Benzyloxycarbonylamino-1-methyl-3-(methylcarbamoyl)azetidine-1-oxide
Abstract
1. Introduction
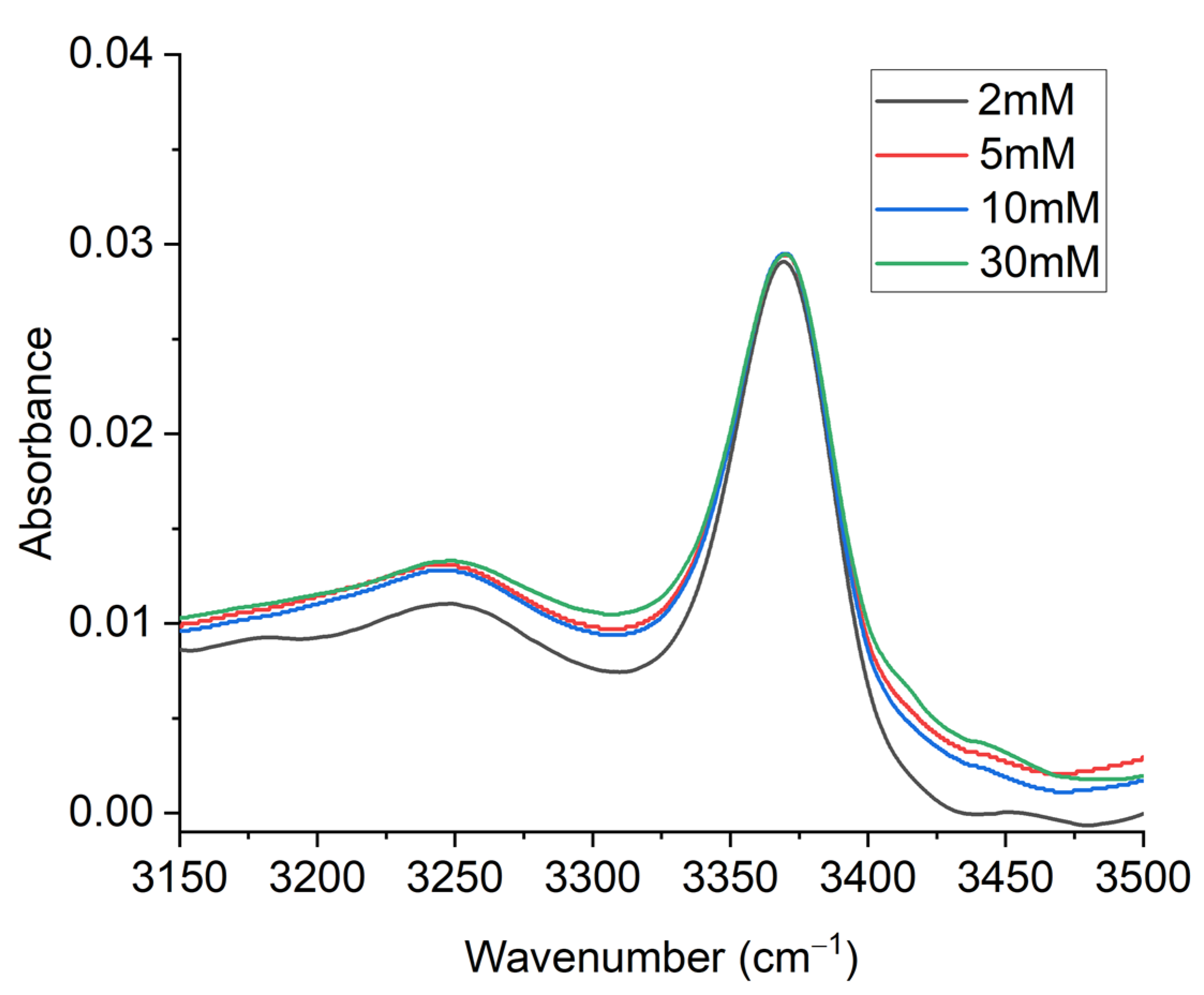
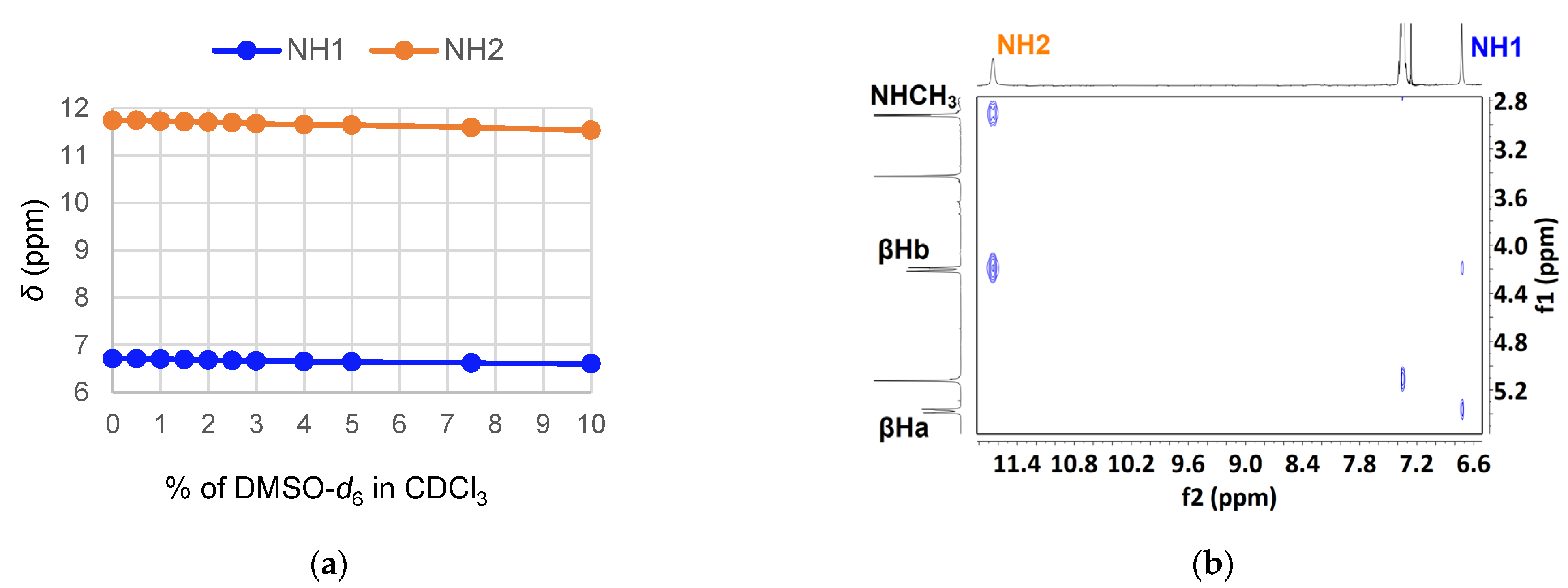
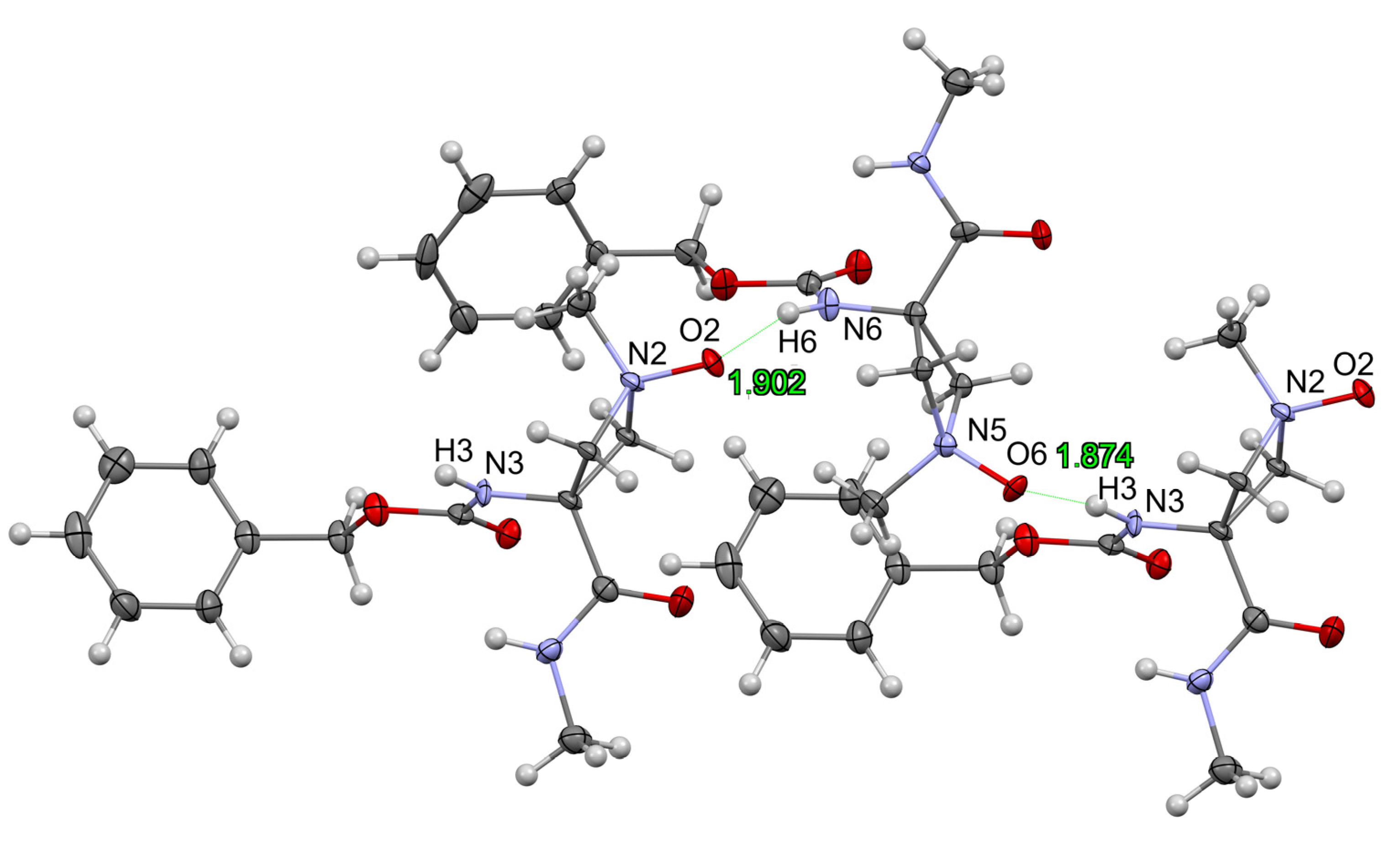
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of trans-Cbz-Aatc(Me,O)-NHMe, 5
3.3. Single Crystal XRD Study of 5
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habib, I.; Singa, K.; Hossain, M. Recent Progress on Pyridine N-Oxide in Organic Transformations: A Review. Chem. Select 2023, 8, e202204099. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Tesenko, F.E. Five-Membered Hetarene N-Oxides: Recent Advances in Synthesis and Reactivity. Synthesis 2021, 53, 3673–3682. [Google Scholar] [CrossRef]
- Wrzeszcz, Z.; Siedlecka, R. Heteroaromatic N-Oxides in Asymmetric Catalysis: A Review. Molecules 2020, 25, 330. [Google Scholar] [CrossRef] [PubMed]
- Bernier, D.; Wefelscheid, U.K.; Woodward, S. Properties, Preparation and Synthetic Uses of Amine N-Oxides. An Update. Org. Prep. Proced. Int. 2009, 41, 173–210. [Google Scholar] [CrossRef]
- O’Neil, I.A. Product Class 3: Amine N-Oxides. In Science of Synthesis; Schaumann, E., Ed.; Thieme: Stuttgart, Germany, 2008; Volume 40b, pp. 855–891. [Google Scholar]
- Albini, A. Synthetic Utility of Amine N-Oxides. Synthesis 1993, 1993, 263–277. [Google Scholar] [CrossRef]
- Menguy, L.; Drouillat, B.; Marrot, J.; Couty, F. Mechanistic Insights into the Rearrangement of Azetidine N-oxides to Isoxazolidines. Tetrahedron Lett. 2012, 53, 4697–4699. [Google Scholar] [CrossRef]
- Yoneda, R.; Araki, L.; Harusawa, S.; Kurihara, T. Studies on the [2,3]-Meisenheimer Rearrangement of 2-Vinylazetidine N-Oxides. Chem. Pharm. Bull. 1998, 46, 853–856. [Google Scholar] [CrossRef][Green Version]
- Kurihara, T.; Sakamoto, Y.; Tsukamoto, K.; Ohishi, H.; Harusawa, S.; Yoneda, R. Meisenheimer Rearrangemlent of Azetopyridoindoles. Part 4. Ring Expansion of 2-Vinyl-1,2,4,10,10a-hexahydroazeto[1′,2′:1,2]pyrido[3,4-b]indole N-oxides. J. Chem. Soc. Perkin Trans. 1 1993, 1993, 81–87. [Google Scholar] [CrossRef]
- Kurihara, T.; Sakamoto, Y.; Takai, M.; Ohuchi, K.; Harusawa, S.; Yoneda, R. Meisenheimer Rearrangement of Azetopyridoindoles. III. Synthesis of 3,6-Epoxyhexahydroazocino[5,4-b]indoles. Chem. Pharm. Bull. 1993, 41, 1221–1225. [Google Scholar] [CrossRef][Green Version]
- O’Neil, I.A.; Potter, A.J. Simple Azetidine N-Oxides: Synthesis, Structure and Reactivity. Chem. Commun. 1998, 1998, 1487–1488. [Google Scholar] [CrossRef]
- O’Neil, I.A.; Miller, N.D.; Barkley, J.V.; Low, C.M.R.; Kalindjian, S.B. Homochiral Proline N-Oxides as Conformational Constraints in Proline Like Molecules. Synlett 1995, 1995, 619–621. [Google Scholar] [CrossRef]
- O’Neil, I.A.; Miller, N.D.; Barkley, J.V.; Low, C.M.R.; Kalindjian, S.B. The Synthesis of Proline Derived Homochiral Amine Oxides. Synlett 1995, 1995, 617–618. [Google Scholar] [CrossRef]
- O’Neil, I.A.; Miller, N.D.; Peake, J.; Barkley, J.V.; Low, C.M.R.; Kalindjian, S.B. The Novel Use of Proline Derived Amine Oxides in Controlling Amide Conformation. Synlett 1993, 1993, 515–518. [Google Scholar] [CrossRef]
- Mundlapati, V.R.; Imani, Z.; D’mello, V.C.; Brenner, V.; Gloaguen, E.; Baltaze, J.-P.; Robin, S.; Mons, M.; Aitken, D.J. N-H···X interactions stabilize intra-residue C5 hydrogen bonded conformations in heterocyclic α-amino acid derivatives. Chem. Sci. 2021, 12, 14826–14832. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Imani, Z.; Gourson, C.; Guillot, R.; Robin, S.; Aitken, D.J. A Post-Synthetic Modification Strategy for the Preparation of Homooligomers of 3-Amino-1-methylazetidine-3-carboxylic acid. Synlett 2023, 34, 1787–1790. [Google Scholar] [CrossRef]
- Liu, D.; Bardaud, J.-X.; Imani, Z.; Robin, S.; Gloaguen, E.; Brenner, V.; Aitken, D.J.; Mons, M. Length-Dependent Transition from Extended to Folded Shapes in Short Oligomers of an Azetidine-Based α-Amino Acid: The Critical Role of NH···N H-Bonds. Molecules 2023, 28, 5048. [Google Scholar] [CrossRef] [PubMed]
- Perrin, D.D.; Armarego, W.L.F. Purification of Laboratory Chemicals, 3rd ed.; Pergamon: Oxford, UK, 1988; p. 123. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Guillot, R.; Robin, S.; Aitken, D.J. trans-3-Benzyloxycarbonylamino-1-methyl-3-(methylcarbamoyl)azetidine-1-oxide. Molbank 2023, 2023, M1726. https://doi.org/10.3390/M1726
Liu D, Guillot R, Robin S, Aitken DJ. trans-3-Benzyloxycarbonylamino-1-methyl-3-(methylcarbamoyl)azetidine-1-oxide. Molbank. 2023; 2023(3):M1726. https://doi.org/10.3390/M1726
Chicago/Turabian StyleLiu, Dayi, Régis Guillot, Sylvie Robin, and David J. Aitken. 2023. "trans-3-Benzyloxycarbonylamino-1-methyl-3-(methylcarbamoyl)azetidine-1-oxide" Molbank 2023, no. 3: M1726. https://doi.org/10.3390/M1726
APA StyleLiu, D., Guillot, R., Robin, S., & Aitken, D. J. (2023). trans-3-Benzyloxycarbonylamino-1-methyl-3-(methylcarbamoyl)azetidine-1-oxide. Molbank, 2023(3), M1726. https://doi.org/10.3390/M1726




