Synthesis of N1-(3,5-Bis(trifluoromethyl)benzyl)benzene-1,2-diamine and N,N-Bis(2-nitrophenyl)-3,5-bis(trifluoromethyl)aniline
Abstract
:1. Introduction
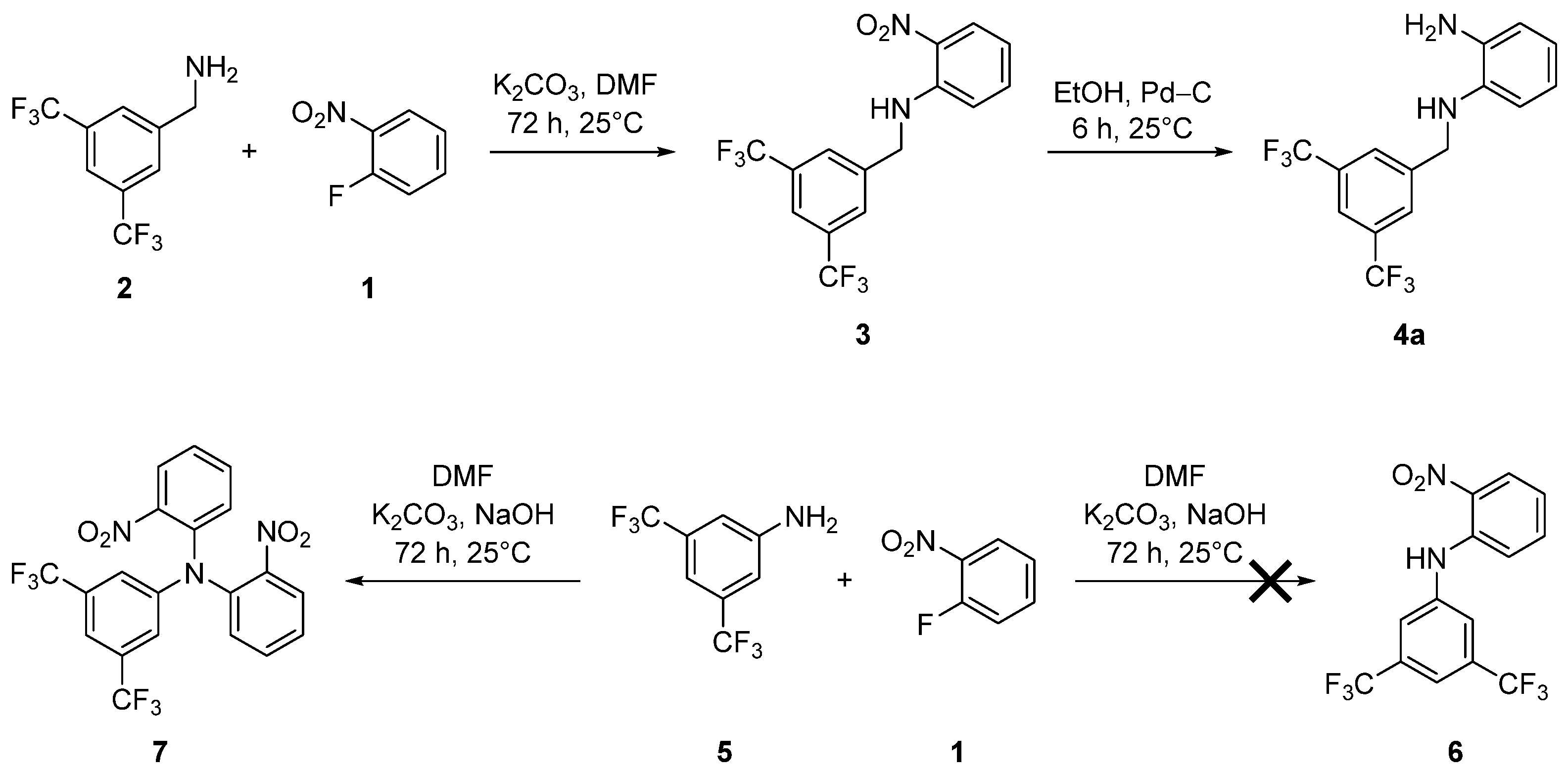
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of N-(3,5-Bis(trifluoromethyl)benzyl)-2-nitroaniline (3)
3.2. Synthesis of N1-(3,5-Bis(trifluoromethyl)benzyl)benzene-1,2-diamine (4a)
3.3. Synthesis of N,N-Bis(2-nitrophenyl)-3,5-bis(trifluoromethyl)aniline (7)
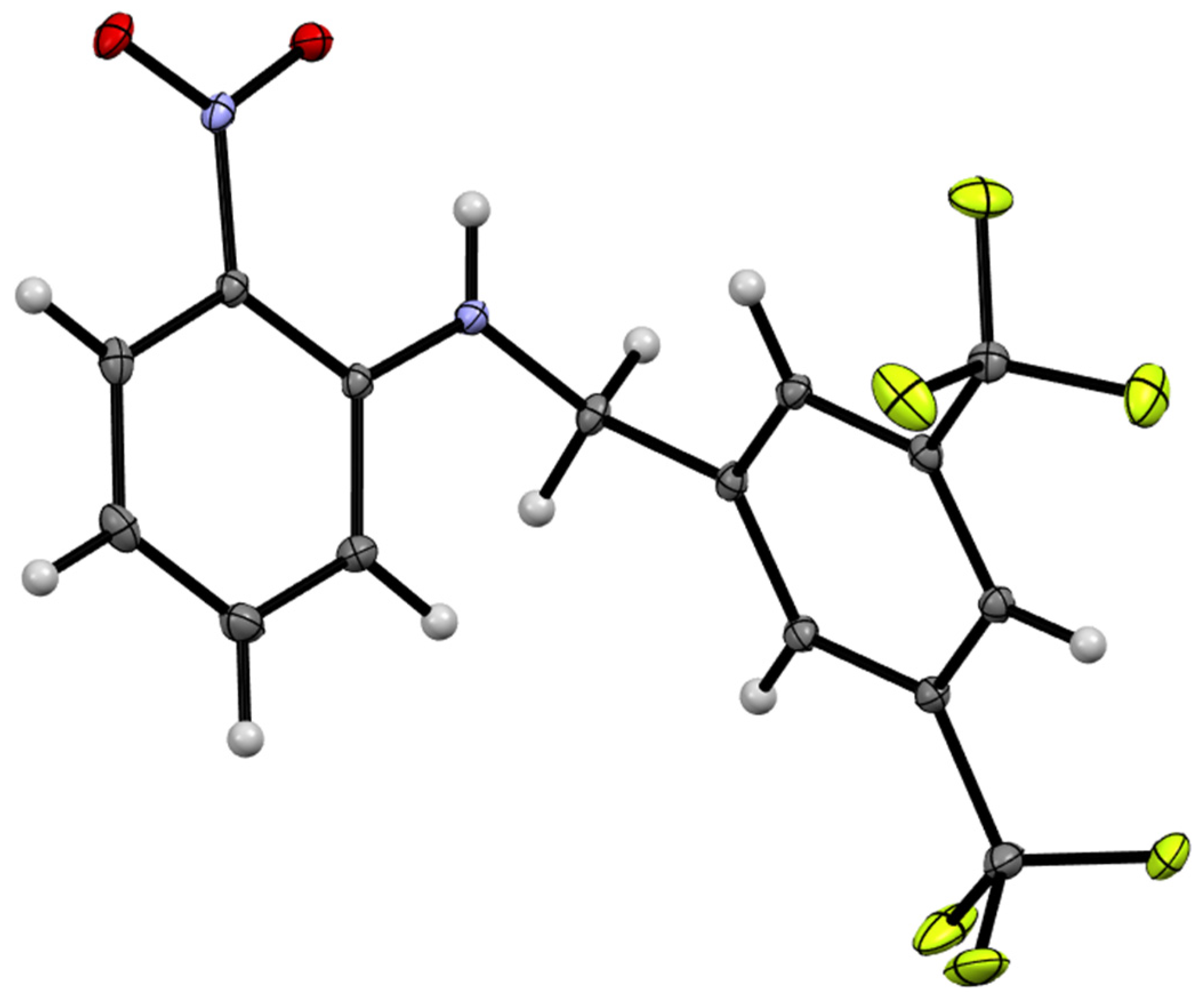
3.4. X-ray Crystallography
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, Y.S.; Kim, M.S.; Kim, Y.C.; Lee, H.; Gao, C.; Ahn, K.-H. A New Blue Light-Emitting Material with Phenylbenzimidazole Moiety and Its Electroluminescence Properties. Mol. Cryst. Liq. Cryst. 2009, 513, 311–319. [Google Scholar] [CrossRef]
- Lai, M.-Y.; Chen, C.-H.; Huang, W.-S.; Lin, J.T.; Ke, T.-H.; Chen, L.-Y.; Tsai, M.-H.; Wu, C.-C. Benzimidazole/Amine-Based Compounds Capable of Ambipolar Transport for Application in Single-Layer Blue-Emitting OLEDs and as Hosts for Phosphorescent Emitters. Angew. Chem. Int. Ed. 2008, 47, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zheng, S.; Bottger, R.; Chae, H.S.; Tanaka, T.; Li, S.; Mochizuki, A.; Jabbour, G.E. Efficient Fluorescent Deep-Blue and Hybrid White Emitting Devices Based on Carbazole/Benzimidazole Compound. J. Phys. Chem. C 2011, 115, 14347–14352. [Google Scholar] [CrossRef]
- Danelzik, T.; Joseph, S.; Mück-Lichtenfeld, C.; Daniliuc, C.G.; Mancheño, O.G. Benzotriazolium Salts: Emergent Readily Accessible Bench-Stable Lewis Acid Catalysts. Org. Lett. 2022, 24, 6105–6110. [Google Scholar] [CrossRef]
- Gampe, D.M.; Kaufmann, M.; Jakobi, D.; Sachse, T.; Presselt, M.; Beckert, R.; Gçrls, H. Stable and Easily Accessible Functional Dyes: Dihydrotetraazaanthracenes as Versatile Precursors for Higher Acenes. Chem. Eur. J. 2015, 21, 7571–7581. [Google Scholar] [CrossRef] [PubMed]
- Hue, B.T.B.; Nguyen, H.M.; Van Hieu, M.; Thanh, D.L.D.; Son, N.H.; De, T.Q.; Morita, H. Facile sodium metabisulfite mediated synthesis of 1,2-disubstituted benzimidazoles and cytotoxicity evaluation. Heterocycles 2019, 98, 650–665. [Google Scholar] [CrossRef]
- Olyaei, A.; Sadeghpour, M. A review on lawsone-based benzo[a]phenazin-5-ol: Synthetic approaches and reactions. RSC Adv. 2022, 12, 13837–13895. [Google Scholar] [CrossRef] [PubMed]
- Ciber, L.; Požgan, F.; Brodnik, H.; Štefane, B.; Svete, J.; Grošelj, U. Synthesis and Catalytic Activity of Organocatalysts Based on Enaminone and Benzenediamine Hydrogen Bond Donors. Catalysts 2022, 12, 1132. [Google Scholar] [CrossRef]
- Hsiaoa, S.-H.; Wu, C.-N. Solution-processable and electroactive aromatic polyamides with 3,5-bis(trifluoromethyl)triphenylamine moiety. Polym. Int. 2017, 66, 916–924. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, L.; Yao, H.; Tian, Y.; Zhu, S.; Guan, S. Novel Hyperbranched Polyimides Bearing Bis(trifluoromethyl)-triphenylamine Moiety: Preparation and Rewritable Nonvolatile Memory Behaviours. ChemistrySelect 2021, 6, 2516–2522. [Google Scholar] [CrossRef]
- Constantina, C.-P.; Damaceanua, M.-D.; Brumaa, M.; Begunovb, R.S. Ortho-CATENATION and trifluoromethyl graphting as driving forces in electro-optical properties modulation of ethanol soluble triphenylamine-based polyimides. Dyes Pigm. 2019, 163, 126–137. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, S.Y. Synthesis of Poly(arylene ether)s Containing Triphenylamine Units via Nitro Displacement Reaction. Macromolecules 2005, 38, 5844–5845. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wu, C.-N. Synthesis and Properties of Redox-Active Polyimides with 3,5-Bis (trifluoromethyl)- or 3,5-Dimethyl-Substituted Triphenylamine Groups. Polym.-Plast. Technol. Mater. 2017, 56, 1274–1285. [Google Scholar] [CrossRef]
- Song, G.; Wang, L.; Liu, D.; Yao, J.; Cao, Y. Gas transport properties of polyimide membranes based on triphenylamine unit. High Perform. Polym. 2018, 30, 100–108. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wu, C.-N. Synthesis and Properties of Fully Triphenylamine-based Polyamides Bearing 3,5-bis(Trifluoromethyl) and/or 3,5-dimethyl Substituents on the Pendent Phenyl Units. Polym.-Plast. Technol. Mater. 2017, 56, 1236–1246. [Google Scholar] [CrossRef]
- Ciber, L.; Požgan, F.; Svete, J.; Štefane, B.; Grošelj, U. 1-{(1S,2S,4R)-7,7-Dimethyl-1-[(pyrrolidin-1-yl)methyl]bicyclo[2.2.1]heptan-2-yl}-1H-benzo[d]imidazole. Molbank 2023, 2023, M1538. [Google Scholar] [CrossRef]
- Ričko, S.; Svete, J.; Štefane, B.; Perdih, A.; Golobič, A.; Meden, A.; Grošelj, U. 1,3-Diamine-Derived Bifunctional Organocatalyst Prepared from Camphor. Adv. Synth. Catal. 2016, 358, 3786–3796. [Google Scholar] [CrossRef]
- Kirsch, P.; Schönleben-Janas, A.; Schirmer, R.H. Synthesis and Characterization of Water-Soluble and Photolabile10-Arylisoalloxazines: Tools for Studying the Mechanism of Action of Flavin-Type Antimalarials. Liebigs Ann. 1995, 1995, 1275–1281. [Google Scholar] [CrossRef]
- Lewin, G.; Schaeffer, C. One-Pot Access to 2,3-Disubstituted 1,2,3,4-Tetrahydroquinolines by Reductive Amination of Aldehydes with Sodium Cyanoborohydride. Heterocycles 1998, 48, 171–174. [Google Scholar] [CrossRef]
- CrysAlis PRO; Agilent Technologies UK Ltd.: Yarnton, UK, 2011.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cristallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciber, L.; Brodnik, H.; Požgan, F.; Svete, J.; Štefane, B.; Grošelj, U. Synthesis of N1-(3,5-Bis(trifluoromethyl)benzyl)benzene-1,2-diamine and N,N-Bis(2-nitrophenyl)-3,5-bis(trifluoromethyl)aniline. Molbank 2023, 2023, M1718. https://doi.org/10.3390/M1718
Ciber L, Brodnik H, Požgan F, Svete J, Štefane B, Grošelj U. Synthesis of N1-(3,5-Bis(trifluoromethyl)benzyl)benzene-1,2-diamine and N,N-Bis(2-nitrophenyl)-3,5-bis(trifluoromethyl)aniline. Molbank. 2023; 2023(3):M1718. https://doi.org/10.3390/M1718
Chicago/Turabian StyleCiber, Luka, Helena Brodnik, Franc Požgan, Jurij Svete, Bogdan Štefane, and Uroš Grošelj. 2023. "Synthesis of N1-(3,5-Bis(trifluoromethyl)benzyl)benzene-1,2-diamine and N,N-Bis(2-nitrophenyl)-3,5-bis(trifluoromethyl)aniline" Molbank 2023, no. 3: M1718. https://doi.org/10.3390/M1718
APA StyleCiber, L., Brodnik, H., Požgan, F., Svete, J., Štefane, B., & Grošelj, U. (2023). Synthesis of N1-(3,5-Bis(trifluoromethyl)benzyl)benzene-1,2-diamine and N,N-Bis(2-nitrophenyl)-3,5-bis(trifluoromethyl)aniline. Molbank, 2023(3), M1718. https://doi.org/10.3390/M1718







