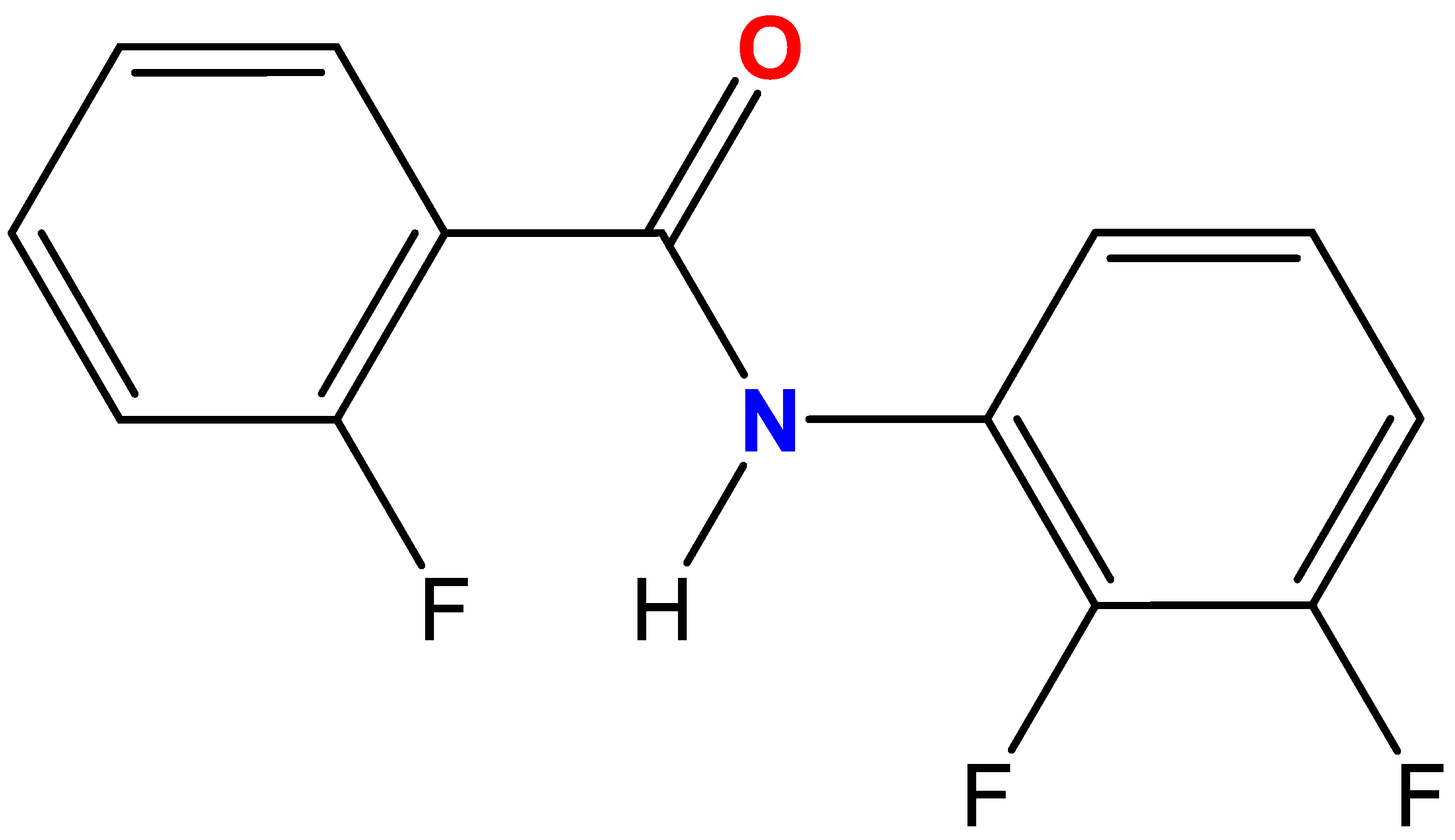
N-(2,3-Difluorophenyl)-2-fluorobenzamide
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Characterisation
2.2. Reaction Procedure and Characterisation: Experimental and Spectroscopic Data
3. Results and Discussion
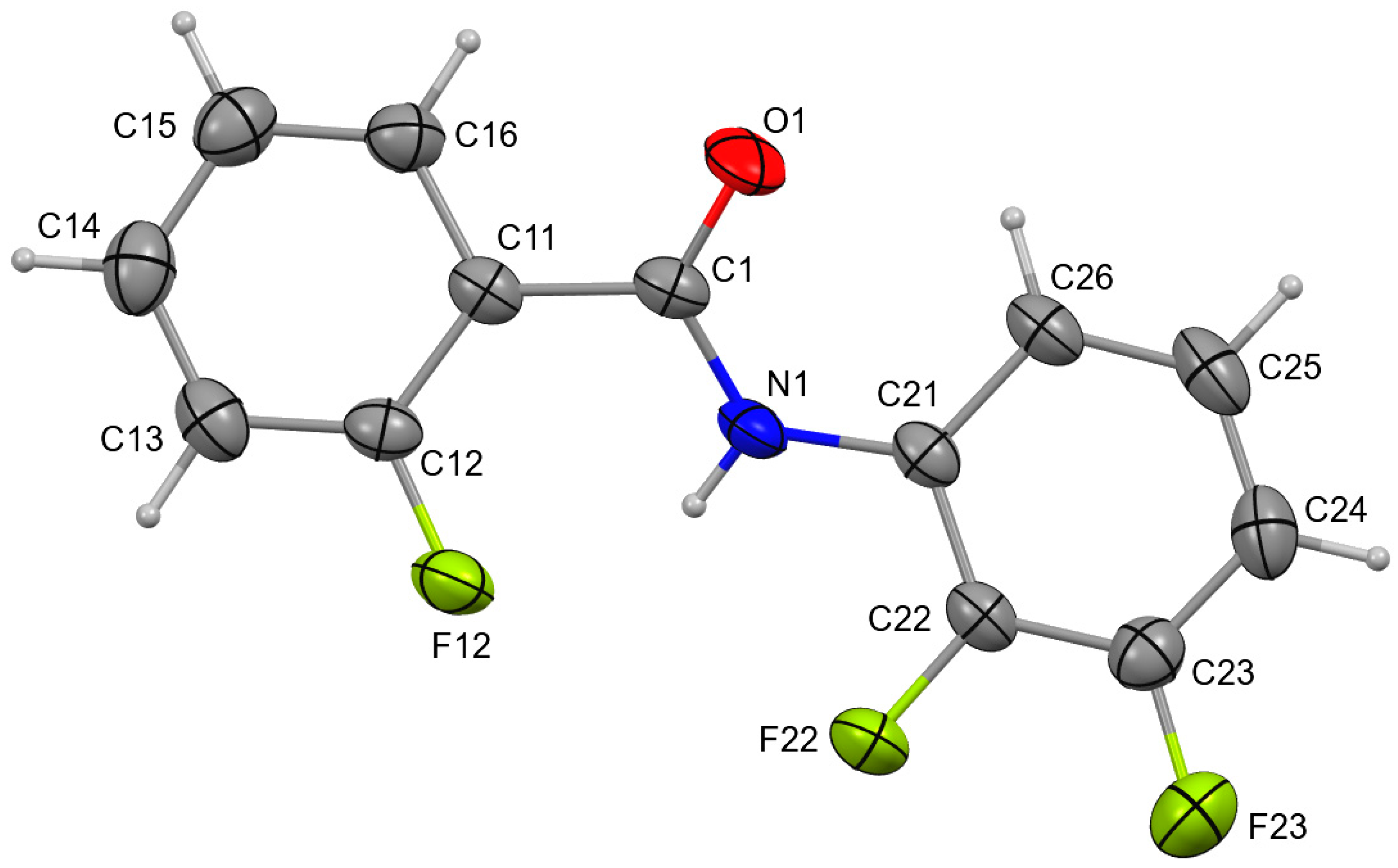
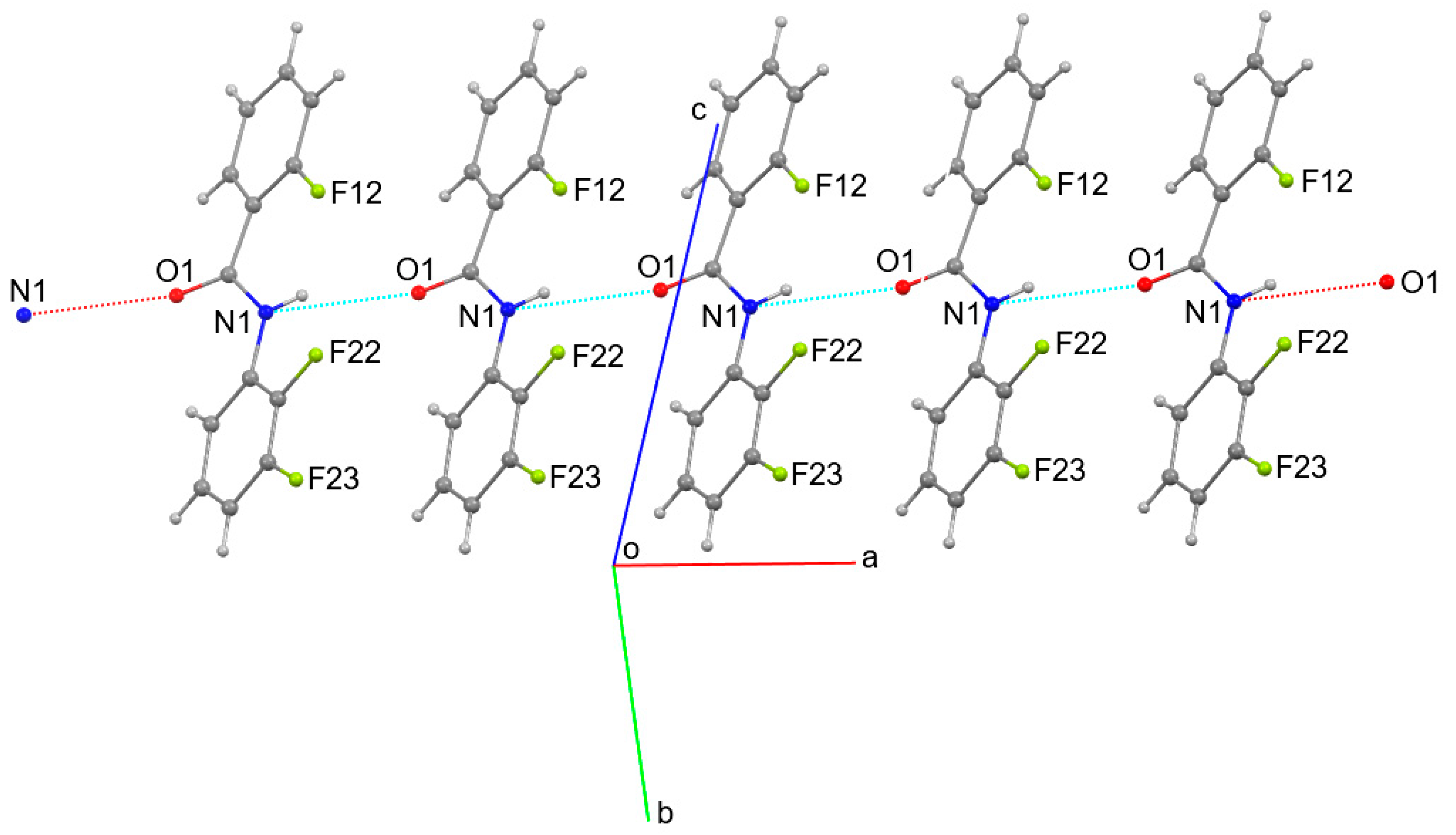
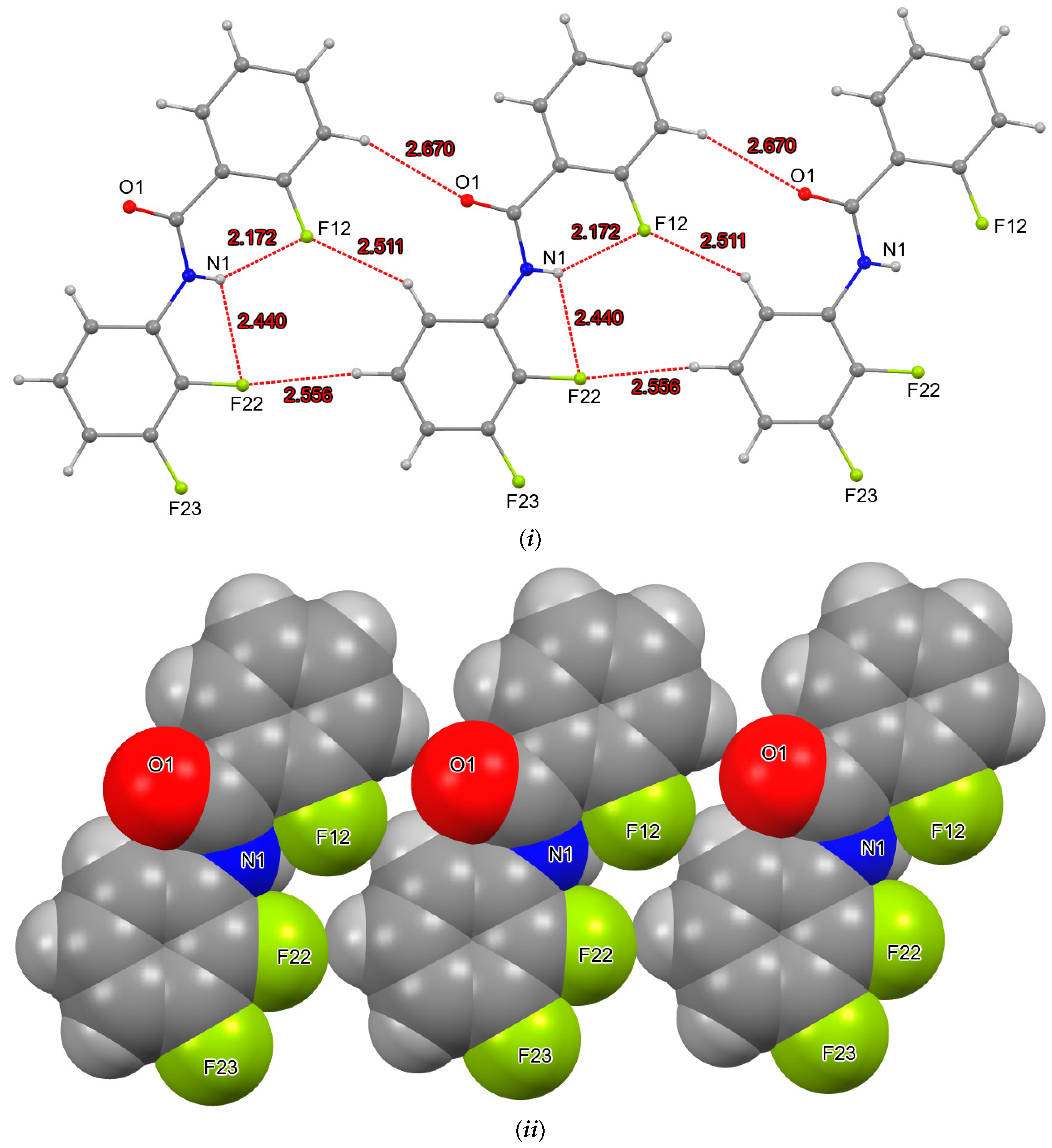
4. Overall Structural Results and Related Literature
5. Conclusions and Future Work
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szostak, M. Amide Bond Activation; Molecules; MDPI: Basel, Switzerland, 2019; Volume 2, pp. 914–928. [Google Scholar] [CrossRef]
- Meng, G.; Zhang, J.; Szostak, M. Acyclic Twisted Amides. Chem. Rev. 2021, 121, 12746–12783. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Zhang, D.W.; Zhao, X.; Hu, J.L.; Li, Z.T. Aromatic Amide Foldamers: Structures, Properties, and Functions. Chem. Rev. 2012, 112, 5271–5316. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Wu, J.; Li, C.; Zhu, J.; Hou, J.L.; Li, C.H.; Jiang, X.K.; Li, Z.T. F⋯H-N and MeO⋯H-N Hydrogen-Bonding in the Solid States of Aromatic Amides and Hydrazides: A Comparison Study. Cryst. Growth Des. 2007, 7, 1490–1496. [Google Scholar] [CrossRef]
- Hagmann, W.K. The Many Roles of Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in Pharmaceuticals: Looking beyond Intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef]
- Chopra, D.; Row, T.G. Evaluation of the interchangeability of C–H and C–F groups: Insights from crystal packing in a series of isomeric fluorinated benzanilides. CrystEngComm 2008, 10, 54–67. [Google Scholar] [CrossRef]
- Mondal, P.K.; Chopra, D. Crystal structure landscape of conformationally flexible organo-fluorine compounds. CrystEngComm 2016, 18, 48–53. [Google Scholar] [CrossRef]
- Mondal, P.K.; Yadav, H.R.; Choudhury, A.R.; Chopra, D. Quantitative characterization of new supramolecular synthons involving fluorine atoms in the crystal structures of di- and tetrafluorinated benzamides. Acta Crystallogr. 2017, B73, 805–819. [Google Scholar] [CrossRef]
- Khavasi, H.R.; Tehrani, A.A. Effect of halogen bonding interaction on supramolecular assembly of halogen-substituted phenylpyrazines. CrystEngComm 2013, 15, 3222–3235. [Google Scholar] [CrossRef]
- Mocilac, P.; Donnelly, K.; Gallagher, J.F. Structural systematics and conformational analyses of a 3 × 3 isomer grid of fluoro-N-(pyridyl)benzamides: Physicochemical correlations, polymorphism and isomorphous relationships. Acta Crystallogr. 2012, B68, 189–203. [Google Scholar] [CrossRef]
- Osman, I.A.; Mocilac, P.; Gallagher, J.F. Short C–H⋯F interactions involving the 2,5-difluorobenzene group: Understanding the role of fluorine in aggregation and complex C–F/C–H disorder in a 2 × 6 isomer grid. CrystEngComm 2016, 18, 5764–5776. [Google Scholar]
- Mocilac, P.; Gallagher, J.F. Monohalogenated carbamates where hydrogen bonding rules without halogen bonding: Is there a link between poor carbamate crystal growth and Z′ > 1? CrystEngComm 2019, 21, 4048–4062. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, W.; Zhou, W.Q.; Liu, H.H.; Wei, S.H.; Fan, J.F. A new crystal structure and fluorescence property of N-2-fluorobenzoyl-N’-4-tolylthiourea. J. Mol. Struct. 2011, 1004, 74–81. [Google Scholar] [CrossRef]
- Kazim, T.; Siegler, M.A.; Leckta, T. Close Amide NH⋯F Hydrogen Bonding Interactions in 1,8-Disubstituted Naphthalenes. J. Org. Chem. 2020, 85, 6195–6200. [Google Scholar] [CrossRef] [PubMed]
- Gowda, B.T.; Foro, S.; Sowmya, B.P.; Fuess, H. 2-Chloro-N-(2,3-dichlorophenyl)benzamide. Acta Crystallogr. 2008, E64, o1342. [Google Scholar] [CrossRef]
- Mocilac, P.; Gallagher, J.F. Structural systematics and conformational analyses of a 3 × 3 isomer grid of nine N-(tolyl)pyridinecarboxamides and three chlorinated relatives. CrystEngComm 2011, 13, 5354–5366. [Google Scholar] [CrossRef]
- Khalaf, R.A.; Al-Rawashdeh, S.; Sabbah, D.; Sheikha, G.A. Molecular Docking and Pharmacophore Modeling Studies of Fluorinated Benzamides as Potential CETP Inhibitors. Med. Chem. 2017, 13, 239–253. [Google Scholar] [CrossRef]
- Moschner, J.; Stulberg, V.; Fernandes, R.; Huhmann, S.; Leppkes, J.; Koksch, B. Approaches to Obtaining Fluorinated α−Amino Acids. Chem. Rev. 2019, 119, 10718–10801. [Google Scholar] [CrossRef]
- Caron, S. Where does the Fluorine Come From? A Review on the Challenges Associated with the Synthesis of Organofluorine Compounds. Org. Process Res. Dev. 2020, 24, 470–480. [Google Scholar] [CrossRef]
- Dehnen, S.; Schafer, L.L.; Leckta, T.; Togni, A. Fluorine: A Very Special Element and Its Very Special Impacts on Chemistry. J. Org. Chem. 2021, 86, 16213–16219. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J.; Wu, J.; Jin, M.; Li, J.; Jin, S.; Li, W.; Xu, D.; Liu, X.; Xu, G. Rational Design, Synthesis, and Biological Evaluation of Fluorine- and Chlorine-Substituted Pyrazol-5-yl-benzamide Derivatives as Potential Succinate Dehydrogenase Inhibitors. J. Agric. Food Chem. 2022, 70, 7566–7575. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hehir, N.; Gallagher, J.F. N-(2,3-Difluorophenyl)-2-fluorobenzamide. Molbank 2023, 2023, M1717. https://doi.org/10.3390/M1717
Hehir N, Gallagher JF. N-(2,3-Difluorophenyl)-2-fluorobenzamide. Molbank. 2023; 2023(3):M1717. https://doi.org/10.3390/M1717
Chicago/Turabian StyleHehir, Niall, and John F. Gallagher. 2023. "N-(2,3-Difluorophenyl)-2-fluorobenzamide" Molbank 2023, no. 3: M1717. https://doi.org/10.3390/M1717
APA StyleHehir, N., & Gallagher, J. F. (2023). N-(2,3-Difluorophenyl)-2-fluorobenzamide. Molbank, 2023(3), M1717. https://doi.org/10.3390/M1717





