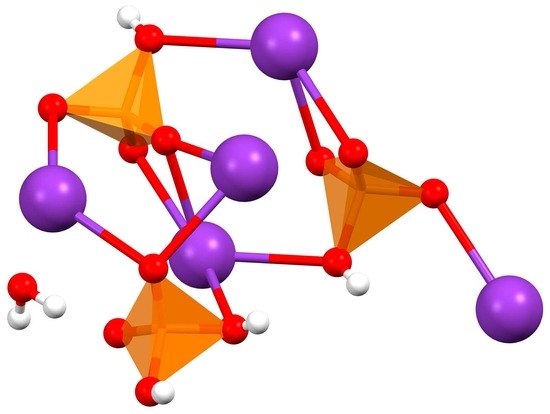
Pentapotassium Bis(hydrogenphospate) Dihydrogenphospate Monohydrate
Abstract
1. Introduction
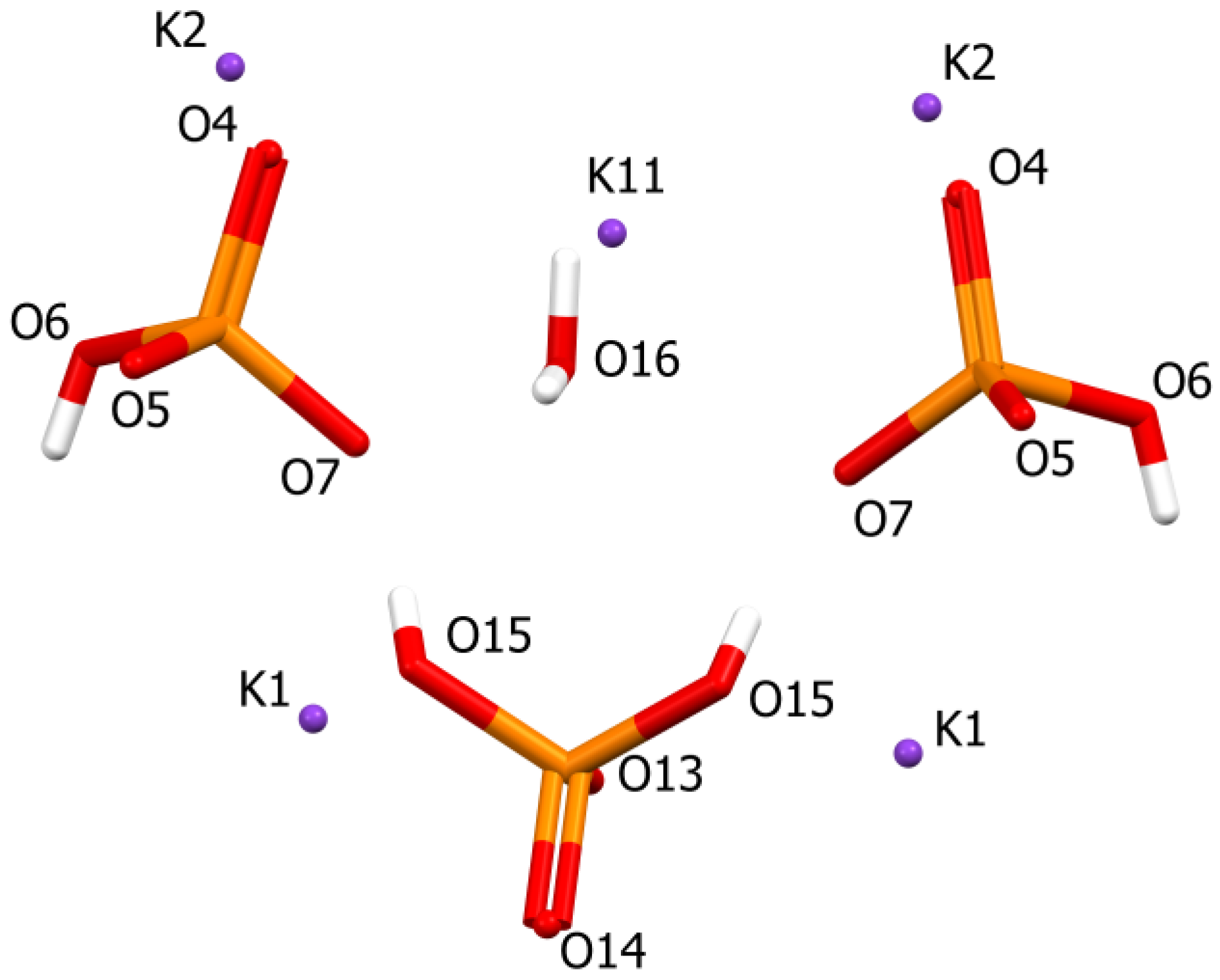
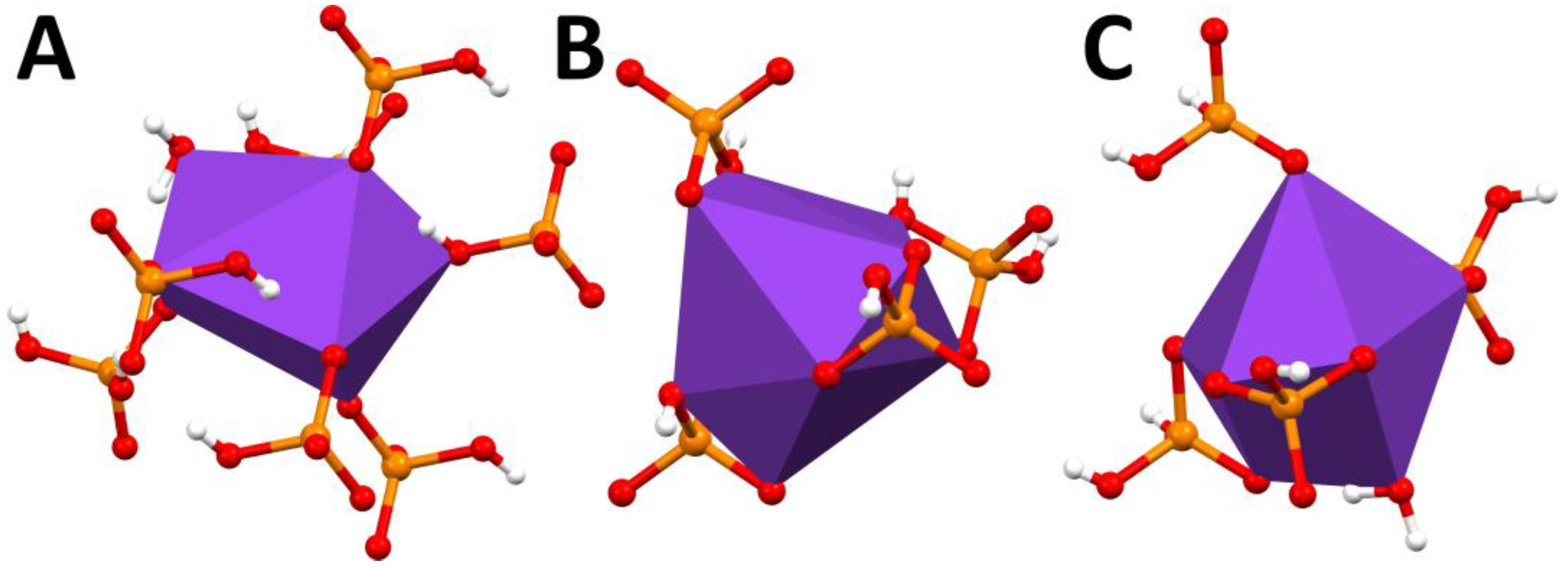
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gomori, G. Preparation of buffers for use in enzyme studies. Meth. Enzymol. 1955, 16, 138–146. [Google Scholar] [CrossRef]
- Hassel, O. Über die Kristallstruktur des primären Kaliumphosphats KH2PO4 und isomorpher Salze. Z. Elektrochem. Angew. Phys. Chem. 1925, 31, 523–529. [Google Scholar]
- Frazer, B.C.; Pepinsky, R. X-ray analysis of the ferroelectric transition in KH2PO4. Acta Cryst. 1953, 6, 273–285. [Google Scholar] [CrossRef]
- Baran, J.; Lis, T.; Ratajczak, H. Structure and polarized IR spectra of the K2HPO4·3H2O crystal. J. Mol. Struct. 1989, 195, 159–174. [Google Scholar] [CrossRef]
- Lis, T. Isomorphous crystals: K2HPO4 and K5Na(HPO4)3. Acta Cryst. 1994, C50, 484–487. [Google Scholar] [CrossRef]
- Mathew, M.; Wong-Ng, W. Crystal Structure of a New Monoclinic Form of Potassium Dihydrogen Phosphate Containing Orthophosphacidium Ion, [H4PO4]+1. J. Solid State Chem. 1995, 114, 219–223. [Google Scholar] [CrossRef]
- Thang Pham, C.; Barnard, I.; Nguyen, H.H.; Abram, U.; Koch, K.R. Cobalt(III) Metallacryptates and Their Guest Cation-Exchange in Solution Monitored by 59Co NMR. Inorg. Chem. 2020, 59, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro, version 1.171.37.35; Rigaku Oxford Diffraction; Rigaku Corporation: Wroclaw, Poland, 2014.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
| Empirical formula | H6 K5 O13 P3 |
| Formula weight | 502.46 |
| Temperature (K) | 297(2) |
| Wavelength (Å) | 0.71073 |
| Crystal system | Monoclinic |
| Space group | P21/m |
| Unit cell dimensions (Å,°) | a = 5.7719(14) |
| b = 17.711(2) | |
| c = 7.3317(18) | |
| Β = 113.28(3) | |
| Volume (Å3) | 688.5(3) |
| Z | 2 |
| Density (calculated) (g/cm3) | 2.424 |
| Absorption coefficient (mm−1) | 2.006 |
| F(000) | 500 |
| Crystal size (mm3) | 0.40 × 0.25 × 0.20 |
| Theta range for data collection (°) | 3.025 to 26.274 |
| Reflections collected | 5780 |
| Independent reflections | 1408 [R(int) = 0.0625] |
| Completeness to θ = 25.242° (%) | 98.4 |
| Max. and min. transmission | 1.00000 and 0.62462 |
| Data/restraints/parameters | 1408/1/113 |
| Goodness-of-fit on F2 | 1.102 |
| Final R indices [I > 2s(I)] | R1 = 0.0438, wR2 = 0.1208 |
| R indices (all data) | R1 = 0.0505, wR2 = 0.1241 |
| (Max, min)(e.Å−3) | 0.638, −0.653 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido, C.C.; Leyssens, T.; Robeyns, K. Pentapotassium Bis(hydrogenphospate) Dihydrogenphospate Monohydrate. Molbank 2023, 2023, M1711. https://doi.org/10.3390/M1711
Garrido CC, Leyssens T, Robeyns K. Pentapotassium Bis(hydrogenphospate) Dihydrogenphospate Monohydrate. Molbank. 2023; 2023(3):M1711. https://doi.org/10.3390/M1711
Chicago/Turabian StyleGarrido, Camila Caro, Tom Leyssens, and Koen Robeyns. 2023. "Pentapotassium Bis(hydrogenphospate) Dihydrogenphospate Monohydrate" Molbank 2023, no. 3: M1711. https://doi.org/10.3390/M1711
APA StyleGarrido, C. C., Leyssens, T., & Robeyns, K. (2023). Pentapotassium Bis(hydrogenphospate) Dihydrogenphospate Monohydrate. Molbank, 2023(3), M1711. https://doi.org/10.3390/M1711