Abstract
The aim of the work was the synthesis of a new 28-acetylbetulin derivative containing an ester group with a carbon–carbon triple bond in the C3 position. To obtain the title compound, a reaction of 28-acetylbetulin with but-2-ynoic acid was carried out according to the Steglich method. The synthetized compound was fully characterized by analyzing the nuclear magnetic resonance spectra (1H-NMR, 13C-NMR), as well as the heteronuclear single quantum coherence (HSQC), and by conducting a heteronuclear multiple bond coherence (HMBC) experiment. Infrared (IR) spectroscopy and high-resolution mass spectrometry (HRMS) were also performed. Additionally, pharmacokinetic parameters and drug similarity of the studied molecule were calculated using in silico methods.
1. Introduction
Compounds of a natural origin are a valuable source of structures that can be transformed into semisynthetic derivatives with higher activity and better pharmacokinetic parameters. The modification of betulin, a lupane-type pentacyclic triterpene, towards ester derivatives has been repeatedly described as a method of obtaining such substances [1,2,3,4]. Due to differences in the reactivity of the primary (at C28) and secondary (at C3) hydroxyl groups, the betulin esterification reaction can be carried out selectively, yielding a 28-monoester derivative. In its reaction with acetic anhydride, 28-acetylbetulin is formed [5]. The second free hydroxyl group present in the molecule creates the possibility of further transformations, and after obtaining the designed structures, it is relatively easy to perform hydrolysis and unlock the hydroxyl group in C28. This strategy is used in various synthetic procedures. On the other hand, according to literature reports, 28-acetylbetulin derivatives modified at C3 constitute a group of compounds with antibacterial, antiviral and anticancer activity (Figure 1) [6,7,8,9,10].
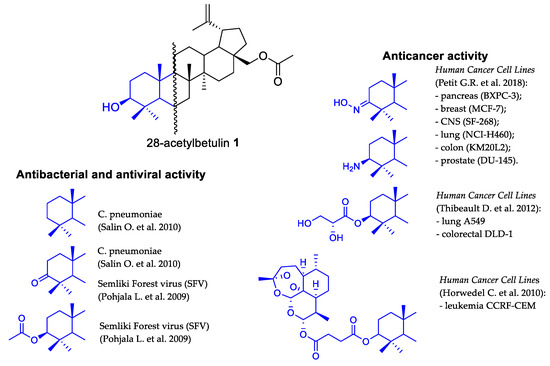
Figure 1.
Different directions of biological activity of 28-acetylbetulin derivatives [6,7,8,9,10].
Many active substances registered as drugs have internal or terminal alkynyl moieties (Figure 2) [11]. This is due to the fact that the unique properties of the triple bond allow them to perform many different functions, directly or indirectly affecting the biological activity of chemical compounds [12].
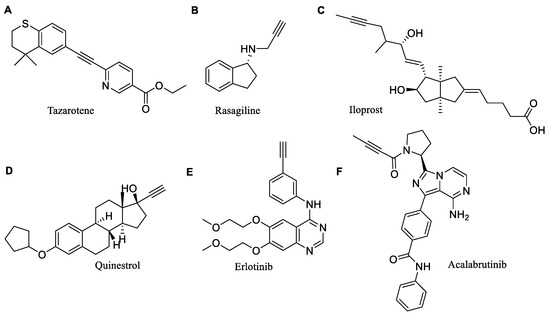
Figure 2.
Examples of drugs containing alkynyl moiety: (A) Tazarotene, (B) Rasagiline, (C) Iloprost, (D) Quinestrol, (E) Erlotinib, (F) Acalabrutinib.
Alkynyl derivatives are used in the pharmacotherapy of various diseases. Tazarotene (Figure 2A) (acne vulgaris and plaque psoriasis) or terbinafine (fungal infections) are effective in treating skin diseases. Rasagiline (Figure 2B) and selegiline have been used in Parkinson’s disease. Iloprost (Figure 2C) and pargyline have an antihypertensive effect. Quinestrol (Figure 2D) is used in hormone replacement therapy to treat menopausal symptoms, such as hot flashes. It is also used to treat breast and prostate cancer. Other anticancer compounds include erlotinib (Figure 2E) for the treatment of certain small cell lung cancers or advanced metastatic pancreatic cancer, and acalabrutinib (Figure 2F) for the treatment of mantle cell lymphoma, chronic lymphocytic leukemia and small lymphocytic lymphoma [11].
Taking into account the above information, we found it interesting to synthesize an alkynyl derivative of diacetyl betulin as well as to initially assess the drug similarity of this molecule.
2. Results and Discussion
2.1. Synthesis of 3-O-But-2-ynoyl-28-O′-acetylbetulin
As previously mentioned, the title compound is a semi-synthetic derivative of betulin. The first step in its preparation was the reaction of betulin with excess acetic anhydride in a dichloromethane solution with the addition of pyridine as a catalyst [5]. The reaction was carried out at room temperature for 24 h. Purification of the crude product by column chromatography produced 28-acetylbetulin 1 with a yield of 77%.
The conversion of the hydroxyl group at the C3 position into a substituent with an alkynyl group was performed by esterification with excess but-2-ynoic acid. The reaction was carried out under mild conditions (Steglich method): initially at −5 °C and then at room temperature, while in the presence of a coupling reagent (N,N’-dicyclohexylcarbodiimide-DCC) and a catalyst (4-dimethylaminopyridine-DMAP). Dried dichloromethane was used as the solvent (Figure 3).
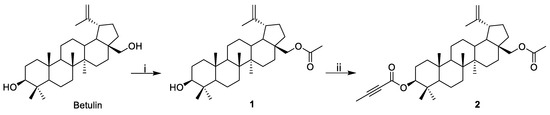
Figure 3.
Synthesis scheme of the target compound: (i) (CH3O)2O, Py, CH2Cl2; (ii) but-2-ynoic acid, DCC, DMAP, CH2Cl2.
2.2. Analysis of the Structure of the Compound 3
The structure of the title compound was confirmed by performing and analyzing the 1H and 13C NMR magnetic resonance spectra (Figures S1 and S2; Supplementary Materials). The location (chemical shift) of the proton signals of the main skeleton of betulin was determined on the basis of data in the literature [13]. The 1H-NMR spectrum shows the basic proton signals of H28 (δH 3.85 ppm and δH 4.18 ppm); H29 (δH 4.59 ppm and δH 4.69 ppm) and H30 (δH 1.69 ppm), characteristic of the isopropenyl group; the multiplet signal of the proton H19 (δH 2.45 ppm); and the five singlet signals of the methyl groups C23, C24, C25, C26 and C27 (δC 0.879; 0.88; 0.85; 1.04 and 0.97 ppm). The numbering of atoms is shown in Figure 4.
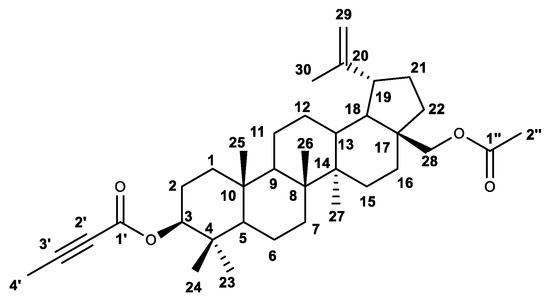
Figure 4.
Structure of compound 2 with carbon numbering.
The most characteristic signals in the 13C-NMR spectrum can be observed at δC 19.1 ppm for C30, δC 109.9 ppm for C29, and δC 150.1 ppm for C20.
The assignment of the signals of the protons and carbon atoms constituting the substituents in positions 3 and 28 and the remaining signals of the structure of the betulin moiety was based on HSQC (Heteronuclear Single Quantum Coherence) and HMBC (Heteronuclear Multiple Bond Correlation) experiments (Figures S3 and S4; Supplementary Materials). The chemical shift of the H3 and two H28 proton signals depends to a greater extent on the substituents present in these positions, and in the spectrum of the tested compound, they are visible, respectively at δH 4.57 ppm (H3), and δH 3.84 ppm and 4.18 ppm. The positions of these atoms have been confirmed by their correlations with carbon atoms at the C3 and C28 position, respectively (HSQC). The HMBC shows that the proton at the H3 position correlated with carbon C1 (δC 38.4 ppm) and C23 (δC 27.9 ppm). In addition, the correlation of H3 with the C1′ (δC 153.9 ppm) atom was observed, which allows for the distinguishing of the carbonyl carbon signals in the ester groups at C3 (C1′) and C28 (C1″ δC 21.0 ppm). Table 1 shows selected proton–carbon correlations.

Table 1.
Selected proton–carbon correlations (HSQC and HMBC experiments) for compound 2 (δ [ppm]—chemical shift of the corresponding signals in the 1H NMR and 13C NMR spectra).
2.3. Physicochemical Characteristics and Selected ADMET Properties of Compound 2
Physicochemical parameters describing the molecule of a chemical compound are commonly used to assess drug similarity and predict the effectiveness of binding to the assumed molecular targets. Properties such as lipophilicity, size, the ability to form hydrogen bonds or undergo ionization affect the absorption, distribution, metabolism, elimination and toxicity (ADMET) profile of potential medicinal substances. The ability to perform a quick initial assessment of a large number of chemical structures means that computational programs designed for this purpose have become a commonly used tool in the search and design of new therapeutics. The rule of five introduced by Lipinski defines drug similarity based on the ranges of physicochemical properties possessed by drugs on the market and is the first criterion for evaluating an orally administered drug candidate [14].
Molinspiration Cheminformatics free web services [15] were used to determine the molecular properties of compound 2, such as log P, topological polar surface area (TPSA), the number of hydrogen bond donors (nHD) and the number of hydrogen bond acceptors (nHA), and molecular weight (MW), as well as violations of Lipinski’s rule of five. Calculations were also made for the precursors of the investigated molecule, which are betulin and 28-acetylbetulin 1. The obtained results are presented in Table 2.

Table 2.
Selected drug similarity parameters of compound 2 and comparison with predicted values for betulin and 28-acetylbetulin 1 as precursors.
None of the tested molecules meet all the criteria of the five rule (MW < 500, lipophilicity log p < 5, number of hydrogen bond donors < 5 and hydrogen bond acceptors < 10) due to high lipophilicity [16]. Compound 2 violates one more criterion of this rule related to the higher molecular weight, but this is not a very big difference, and substances with much higher masses are used in therapy. The parameter values determined by Lipinski are the criteria for the selection of substances with the best solubility in the prediction of oral-drug-likeness. High lipophilicity (often connected with high molecular weight) determines the lower solubility of drugs in liquids, determines the penetration through biological membranes, the rate of drug absorption in the digestive tract, affinity for plasma proteins and tissues, and accumulation in the body [17].
In the case of triterpene derivatives, the greatest limitation of their use as medicinal substances is their poor solubility and thus low bioavailability. For the tested derivatives, parameters related to absorption were selected for the ADMET analysis, i.e., water solubility, Caco-2 permeability, human intestinal absorption, interaction with P-glycoproteins and permeability of the blood–brain barrier (BBB) (Table 2).
Tested derivatives characterized high human intestinal absorption (100%) but poor water solubility. Compounds can be categorized by solubility value expressed as logS into highly soluble (logS equal to 0 and above), soluble (logS 0 to −2), slightly soluble (logS −2 to −4) and insoluble (logS −4) [19]. However, currently, many methods are being developed to circumvent the limitations associated with the poor oral bioavailability of drugs of a natural origin, such as the use of liposomes, micelles, polymer nanoparticles, dendrimers or inorganic nanoparticles [20].
Absorption of the substance through the human intestinal mucosa (study based on Caco-2 cells) for the tested compounds was expressed as a logPapp value in the range of 1.238 to 1.321. It is assumed that a value > 0.9 indicates high Caco-2 permeability [18], so the tested compounds are characterized by good permeability, but the structural changes introduced in betulin do not significantly affect this parameter.
The P-gp transport protein plays a significant role in the active transport of various substances outside of cells. This effect may result in decreased intestinal permeation and decreased bioavailability of orally administered drugs. All compounds tested may have an inhibitory effect on P-glycoprotein. The use of P-gp inhibitor therapy in combination with other drugs that are P-gp substrates may result in drug–drug interactions and side effects [18].
Betulin and its derivatives 1 and 2 are characterized by an average ability to cross the blood–brain barrier. On the other hand, from predicting the toxic effect of these compounds, based on the results obtained, it can be seen that they do not show toxicity in the Ames test, and structural changes have transformed betulin into derivatives that do not show hepatotoxicity.
3. Materials and Methods
3.1. Chemistry
Betulin was obtained by extracting birch bark from trees growing in southern Poland. The crude product was crystallized from ethanol with activated carbon and then from propan-2-ol. All other reagents were purchased from Merck (Darmstadt, Germany) and used without further purification. The monitoring of the reaction progress as well as purity control of the products was carried out using the thin-layer chromatography (TLC) method. TLC analysis was performed on precoated silica gel 60 F254 plates (Merck, Darmstadt, Germany) and visualized by spraying with an ethanolic solution of sulfuric acid and heating. The final product was purified by column chromatography using silica gel 60 (0.063–0.200 mm; Merck, Darmstadt, Germany) as stationary phase and a mixture of chloroform and ethanol 60:1 (v/v) as mobile phase
Melting points were determined using an Electrothermal IA 9300 apparatus (Bibby Scientific Limited, Stone, Southampton, GB, UK) (heating rate of 2 °C/min). 1H and 13C NMR spectra as well as HSQC and HMBC were taken for a sample of compound 2 dissolved in deuterated chloroform. Measurements were made on the Bruker Avance III 600 spectrometer (Bruker, Billerica, MA, USA). IR spectra were obtained using an IRAffinity-1 Shimadzu spectrometer (Shimadzu Corporation, Kyoto, Japan). High-resolution mass spectra (HRMS APCI) were acquired using a Bruker Impact II instrument (Bruker).
3.2. Procedure for Obtaining 3-O-But-2-ynoyl-28-O′-acetylbetulin 2
In a round-bottomed flask, 1 mmol of 28-acetylbetulin 1 was dissolved in 5 mL of dichloromethane. The solution was cooled in an ice-salt bath to −5 °C and then 1.17 mmol of but-2-ynic acid was added. A solution of 240 mg of DCC and 10 mg of DMAP in 1 mL of dichloromethane was slowly added dropwise. The reaction was carried out under an argon atmosphere while stirring with a magnetic stirrer. For the first 5 h, the flask with the reaction mixture was in an ice-salt bath, after which the reaction was carried out at room temperature. After 24 h, the precipitate was filtered off, the filtrate was concentrated to dryness in a vacuum evaporator. The reaction products were purified by column chromatography (SiO2, chloroform ethanol 60:1, v/v).
3-O-But-2-ynoyl-28-O′-acetylbetulin 2. Yield 75%; mp 253–254 °C; Rf 0.65 (chloroform/ethanol, 60:1, v/v); 1H NMR (600 MHz, CDCl3) δ ppm: 0.78 (d, 1H, H5), 0.85 (s, 3H, CH3), 0.879 (s, 3H, CH3), 0.88 (s, 3H, CH3), 1.32 (s, 3H, CH3), 1.69 (s, 3H, CH3), 1.99 (s, 3H, CH3C≡), 2.09 (s, 3H, CH3C=O), 2.45 (m, 1H, H-19), 3.85 (d, J = 11.4 Hz, 1H, H-28), 4.18 (d, J = 10.8 Hz, 1H, H-28), 4.57 (m, 1H, H-3), 4.59 (s, 1H, H-29), 4.69 (s, 1H, H-29) (Figure S1-Supplementary Materials).
13C NMR (150 MHz, CDCl3) δ ppm: 3.9 (C4′), 14.7 (C27), 16.0 (C26), 16.1 (C25), 16.5 (C24), 18.1 (C6), 19.1 (C30), 20.8 (C11), 21.0 (C2″), 23.5 (C2), 25.1 (C12), 27.1 (C13), 27.9 (C23), 34.1 (C7), 34.5 (C22), 37.0 (C10), 37.5 (C13), 37.9 (C4), 38.4 (C1), 40.8 (C8), 42.6 (C14), 46.3 (C17), 47.7 (C19), 48.7 (C18), 50.2 (C9), 55.4 (C5), 62.8 (C28), 72.9 (C2′), 82.8 (C3), 84.9 (C3′), 109.9 (C29), 150.1 (C20), 153.9 (C1′), 170.6 (C1″) (Figure S2-Supplementary Materials); IR (ν max cm−1, KBr): 889; 1256; 1703; 1735; 2239; 3857 (Figure S5-Supplementary Materials).
HR-MS (APCI) m/z (M-H): 549.3949; C35H54O4 (Calculated 549.3944).
3.3. Assessment of ADMET Properties and Drug-Likeness of the Tested Molecules
The structures of the tested compounds were converted into SMILES codes using the ChemDraw application and sent to the free website of Molinspiration Cheminformatics for the calculation of physicochemical parameters and the assessment of drug similarity [15]. The analysis of ADMET parameters was achieved using pkCSM BiosigLab [18].
Supplementary Materials
The following supporting information can be downloaded. Figure S1: 1H-NMR spectrum of compound 2; Figure S2: 13C-NMR spectrum of compound 2; Figure S3: HSQC spectrum of compound 2; Figure S4: HMBC spectrum of compound 2; Table S1: 1H and 13C NMR data for compound 2; Figure S5: IR spectrum of compound 2.
Author Contributions
E.C.: conceptualization, writing—original draft preparation, formal analysis, writing—review and editing, and investigation: E.B.: synthesis, methodology, and writing—review and editing; M.K.-T.: formal analysis and methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Medical University of Silesia in Katowice Grant nos. PCN-1-042/K/2/F and PCN-1-044/K/2/F.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Drąg-Zalesinska, M.; Kulbacka, J.; Saczko, J.; Wysocka, T.; Zabel, M.; Surowiak, P.; Drąg, M. Esters of betulin and betulinic acid with amino acids have improved water solubility and are selectively cytotoxic toward cancer cells. Bioorg. Med. Chem. Lett. 2009, 15, 4814–4817. [Google Scholar] [CrossRef] [PubMed]
- Borkova, L.; Jasikova, L.; Rehulka, J.; Frisonsova, K.; Urban, M.; Frydrych, I.; Popa, I.; Hajduch, M.; Dickinson, N.J.; Dzubak, P.; et al. Synthesis of cytotoxic 2,2-difluoroderivatives of dihydrobetulinic acid and allobetulin and study of their impact on cancer cells. Eur. J. Med. Chem. 2015, 96, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Pęcak, P.; Świtalska, M.; Chrobak, E.; Boryczka, G.; Bębenek, E. Betulin Acid Ester Derivatives Inhibit Cancer Cell Growth by Inducing Apoptosis through Caspase Cascade Activation: A Comprehensive In Vitro and In Silico Study. Int. J. Mol. Sci. 2023, 24, 196. [Google Scholar] [CrossRef] [PubMed]
- Bębenek, E.; Chrobak, E.; Marciniec, K.; Kadela-Tomanek, M.; Trynda, J.; Wietrzyk, J.; Boryczka, S. Biological Activity and In Silico Study of 3-Modified Derivatives of Betulin and Betulinic Aldehyde. Int. J. Mol. Sci. 2019, 20, 1372. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, C.; Legault, J.; Lebrun, M.; Dufour, P.; Pichette, A. Glycosidation of lupane-type triterpenoids as potent in vitro cytotoxic agents. Bioorg. Med. Chem. 2006, 14, 6713–6725. [Google Scholar] [CrossRef] [PubMed]
- Salin, O.; Alakurtti, S.; Pohjala, L.; Siiskonen, A.; Maass, V.; Maass, M.; Yli-Kauhaluoma, J.; Vuorela, P. Inhibitory effect of the natural product betulin and its derivatives against the intracellular bacterium Chlamydia pneumoniae. Biochem. Pharmacol. 2010, 80, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Pohjala, L.; Alakurtti, S.; Ahola, T.; Yli-Kauhaluoma, J.; Tammela, P. Betulin-derived compounds as inhibitors of alphavirus replication. J. Nat. Prod. 2009, 72, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Melody, N.; Chapuis, J.-C. Antineoplastic Agents. 606. The Betulastatins. J. Nat. Prod. 2018, 81, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Thibeault, D.; Gauthier, C.J.; Legault, J.; Bouchard, L.; Gagné, A. Pichette Synthesis and cytotoxicity of lupane-type triterpenoid glyceryl esters. Bioorg. Med. Chem. Lett. 2012, 22, 4735–4739. [Google Scholar] [CrossRef] [PubMed]
- Horwedel, C.; Tsogoeva, S.B.; Wei, S.; Efferth, T. Cytotoxicity of Artesunic Acid Homo- and Heterodimer Molecules toward Sensitive and Multidrug-Resistant CCRF-CEM Leukemia Cells. J. Med. Chem. 2010, 53, 4842–4848. [Google Scholar] [CrossRef] [PubMed]
- DrugBank Online. Available online: https://go.drugbank.com/structures/search/small_molecule_drugs/structure (accessed on 19 May 2023).
- Talele, T.T. Acetylene group, friend or foe in medicinal chemistry. J. Med. Chem. 2020, 63, 5625–5663. [Google Scholar] [CrossRef] [PubMed]
- Lomchid, P.; Nasomjai, P.; Kanokmedhakul, S.; Boonmak, J.; Youngme, S.; Kanokmedhakul, K. Bioactive Lupane and Hopane Triterpenes from Lepisanthes senegalensis. Planta Med. 2017, 83, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R. Theoretical Studies on the Molecular Properties, Toxicity, and Biological Efficacy of 21 New Chemical Entities. ACS Omega 2021, 6, 24891–24901. [Google Scholar] [CrossRef] [PubMed]
- Molinspiration Cheminformatics Free Web Services, Slovensky Grob, Slovakia. Available online: https://www.molinspiration.com (accessed on 23 May 2023).
- Lipinski, C.A. Drug-like Properties and the Causes of Poor Solubility and Poor Permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.; Tom, L.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- pkCSM—Biosig Lab. Available online: https://biosig.lab.uq.edu.au/pkcsm/prediction (accessed on 22 May 2023).
- Sorkun, M.C.; Khetan, A.; Er, S. AqSolDB, a curated reference set of aqueous solubility and 2D descriptors for a diverse set of compounds. Sci. Data 2019, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Madej, M.; Kurowska, N.; Strzalka-Mrozik, B. Polymeric Nanoparticles—Tools in a Drug Delivery System in Selected Cancer Therapies. Appl. Sci. 2022, 12, 9479. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).