3-(2-Chloro-5-methylphenoxy)propane-1,2-diol
Abstract
1. Introduction
2. Results and Discussion
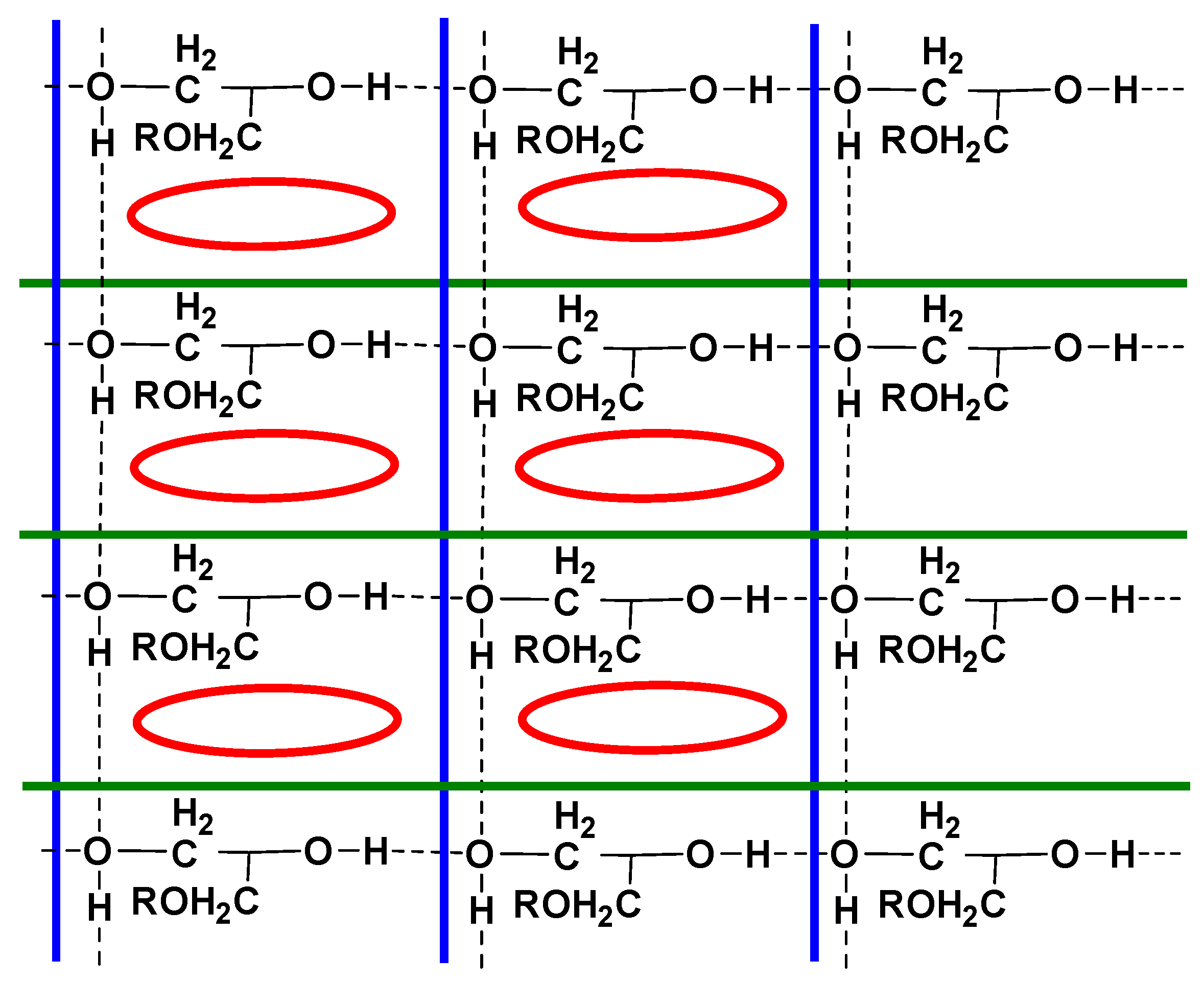
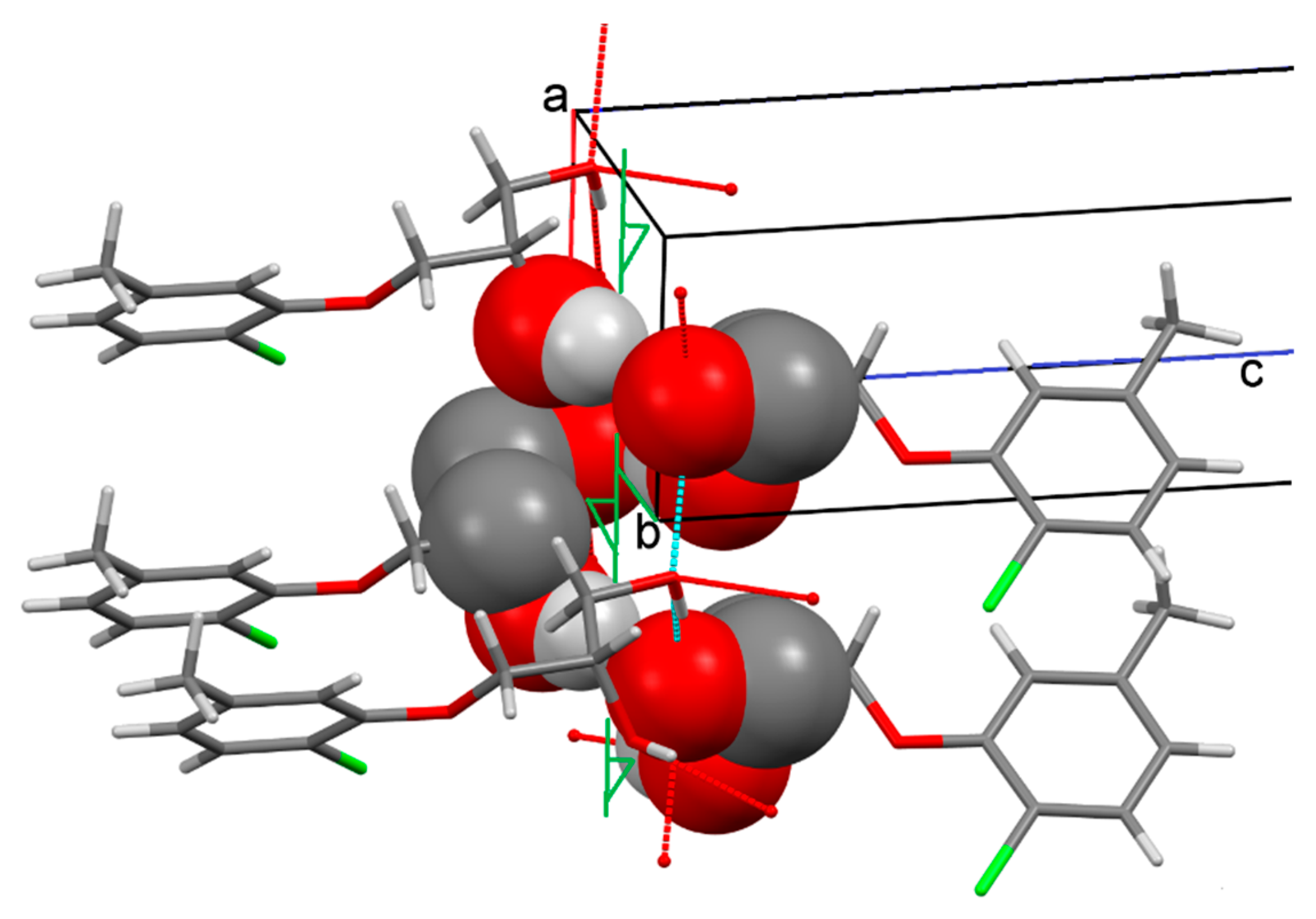
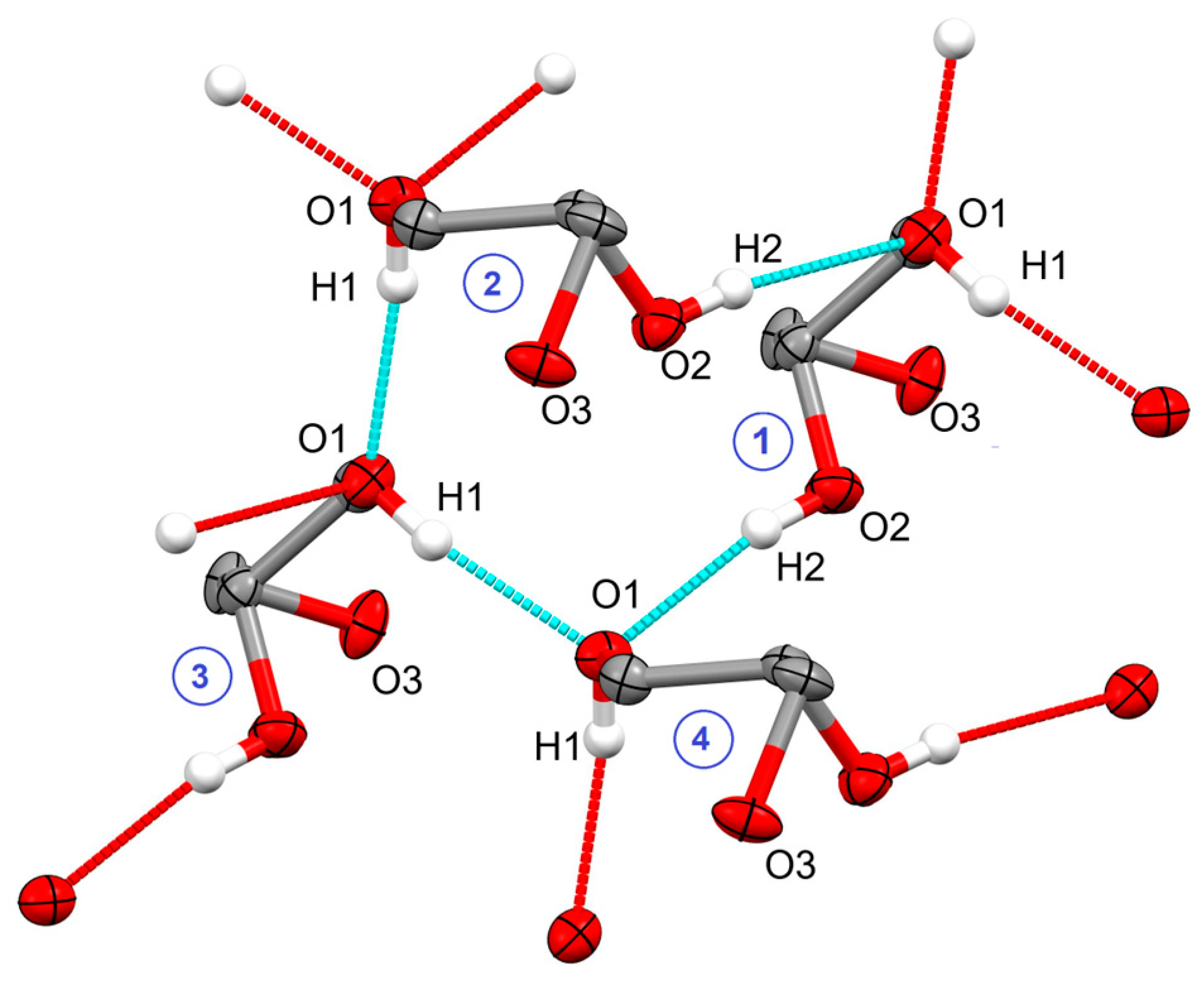
2.1. Crystal Structure of Racemic Diol 1
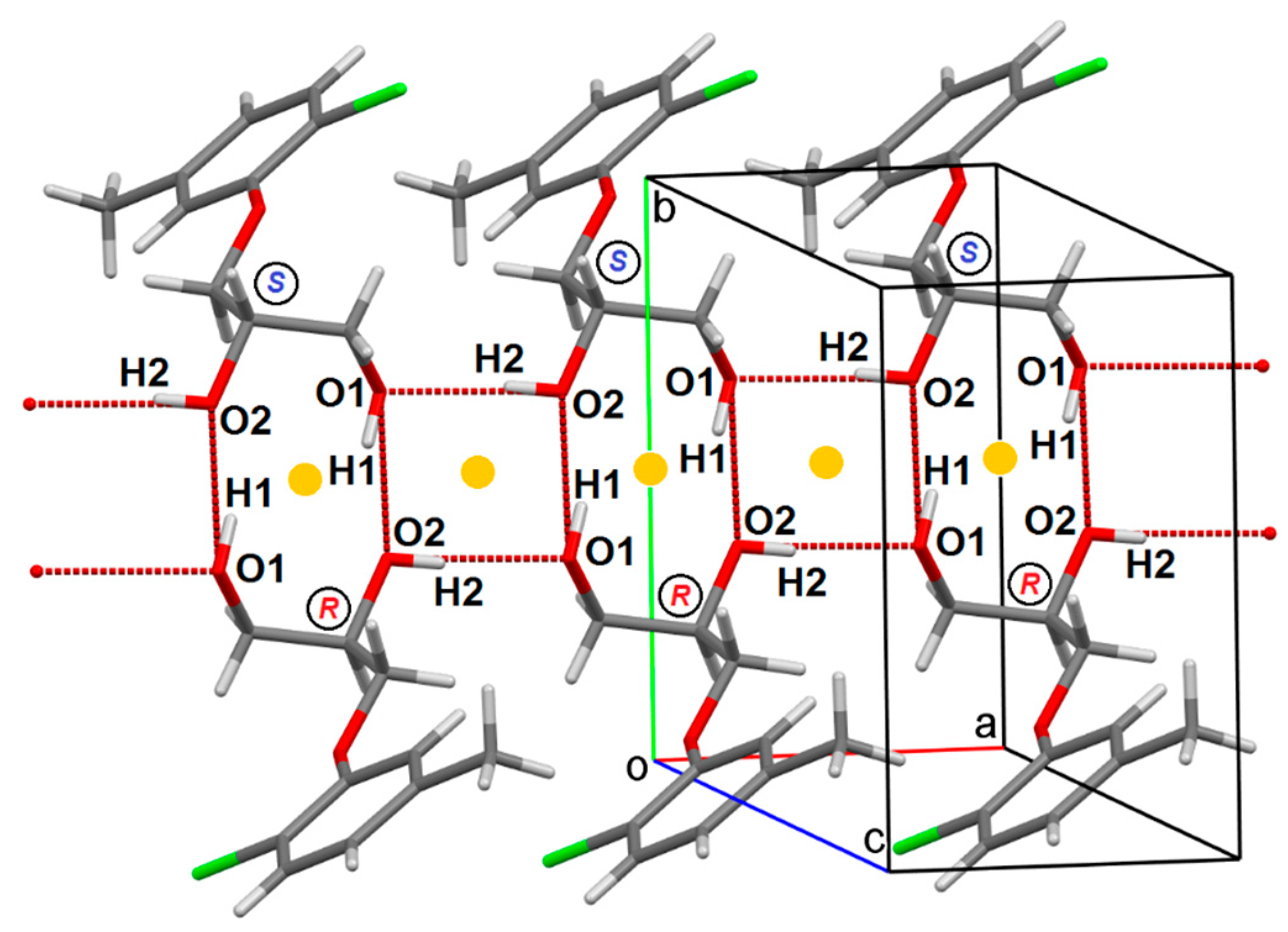
2.2. Crystal Structure of Single-Enantiomeric Diol (S)-1
2.3. Discussion
3. Materials and Methods
3.1. Materials
3.2. Instruments
3.3. Single Crystal X-ray Diffraction
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Crystallographic Data
| Compound | rac-1 | (S)-1 |
| Empirical formula | C10H13ClO3 | C10H13ClO3 |
| Formula weight | 216.65 | 216.65 |
| Temperature (K) | 173(2) | 173(2) |
| Radiation, wavelength (Å) | MoKα, 0.71073 | |
| Crystal system | Monoclinic | Orthorhombic |
| Space group | P21/n (No. 14) | P212121 (No. 19) |
| Unit cell dimensions (Å; deg) | a = 5.4729(9), b = 8.7652(14), c = 21.370(3); β = 96.201(11) | a = 4.5908(6), b = 5.6201(8), c = 39.348(5) |
| Volume (Å3) | 1019.1(3) | 1015.2(2) |
| Z and Z’ | 4 and 1 | 4 and 1 |
| Calculated density (g cm−3) | 1.412 | 1.417 |
| Absorption coefficient (mm−1) | 0.353 | 0.354 |
| F(000) | 456 | 456 |
| Crystal size (mm3) | 0.668 × 0.157 × 0.040 | 0.980 × 0.220 × 0.060 |
| θ range for data collection | 1.917 to 25.050° | 3.106 to 26.994° |
| Index ranges | −6 ≤ h ≤ 6, −10 ≤ k ≤ 10, −25 ≤ l ≤ 25 | −5 ≤ h ≤ 5, −7 ≤ k ≤ 7, −45 ≤ l ≤ 50 |
| Reflections collected/Rint | 10,121/0.0689 | 8199/0.0374 |
| Independent reflections | 1782 | 2188 |
| Observed Data [I > 2σ(I)] | 1352 | 1945 |
| Completeness to θmax (%) | 98.1 | 99.3 |
| Absorption correction | Semi-empirical from equivalents | |
| Max. and min. transmission | 0.6414 and 0.7461 | 0.6394 and 0.7455 |
| Data/restraints/parameters | 1782/0/136 | 2188/0/136 |
| Goodness-of-fit on F2 | 1.062 | 1.060 |
| Final R indices [I > 2σ(I)] | R1 = 0.0573, wR2 = 0.1307 | R1 = 0.0370, wR2 = 0.0650 |
| R indices (all data) | R1 = 0.0804, wR2 = 0.1432 | R1 = 0.0458, wR2 = 0.0683 |
| Flack parameter | - | 0.03(4) |
| Largest diff. peak and hole (e Å−3) | 0.487 and −0.405 | 0.237 and −0.181 |
| Compound | Interaction O–H∙∙∙O′ | Interatomic Distances (Å) | Angle O–H∙∙∙O′ (deg) | Symmetry Operation | ||
|---|---|---|---|---|---|---|
| d(O–H) | d(H∙∙∙O′) | d(O∙∙∙O′) | ||||
| rac-1 | O1–H1∙∙∙O2 | 0.88(5) | 1.94(5) | 2.754(3) | 151(4) | −x + 1, −y + 2, −z |
| O2–H2∙∙∙O1 | 0.81(4) | 1.93(4) | 2.739(3) | 176(4) | x + 1, y, z | |
| (S)-1 | O1–H1∙∙∙O1 | 0.83(3) | 1.92(3) | 2.7412(19) | 168(3) | x + 1/2, −y + 5/2, −z + 2 |
| O2–H2∙∙∙O1 | 0.82(3) | 2.06(3) | 2.866(3) | 170(3) | x + 1/2, −y + 3/2, −z + 2 | |
References
- Merck and Co. The Merck Index, 14th ed.; O’Neil, M.J., Ed.; Merck and Co.: Whitehouse Station, NJ, USA, 2006; p. 245, Entry 1496. [Google Scholar]
- Ding, H.; Liu, S.; Zhao, K.-X.; Pu, J.; Xie, Y.-F.; Zhang, X.-W. Comparative efficacy of antihypertensive agents in flow-mediated vasodilation of patients with hypertension: Network meta-analysis of randomized controlled trial. Int. J. Hypertens. 2022, 2022, 2432567. [Google Scholar] [CrossRef] [PubMed]
- Bredikhin, A.A.; Bredikhina, Z.A.; Kurenkov, A.V.; Zakharychev, D.V.; Gubaidullin, A.T. Synthesis, phase behavior and absolute configuration of β-adrenoblocker bupranolol and related compounds. J. Mol. Struct. 2018, 1173, 157–165. [Google Scholar] [CrossRef]
- Malinowska, B.; Kieć-Kononowicz, K.; Flau, K.; Godlewski, G.; Kozłowska, H.; Kathmann, M.; Schlicker, E. Atypical cardiostimulant β-adrenoceptor in the rat heart: Stereoselective antagonism by bupranolol but lack of effect by some bupranolol analogues. Br. J. Pharmacol. 2003, 139, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Piltonen, M.H.; Gauthier, J.; Convertino, M.; Acland, E.L.; Dokholyan, N.V.; Mogil, J.S.; Diatchenko, L.; Maixner, W. Differences in the antinociceptive effects and cinding properties of propranolol and bupranolol enantiomers. J. Pain 2015, 16, 1321–1333. [Google Scholar] [CrossRef] [PubMed]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic-compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.L. Patterns in hydrogen bonding—Functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Bredikhin, A.A.; Fayzullin, R.R.; Gubaidullin, A.T.; Bredikhina, Z.A. Intermolecular hydrogen bonding in alpha-hydroxy carboxylic acids crystals: Connectivity, synthons, supramolecular motifs. Crystals 2022, 12, 1479. [Google Scholar] [CrossRef]
- Flack, H.D.; Bernardinelli, G. Reporting end evaluating absolute structure and absolute configuration determination. J. Appl. Crystallogr. 2000, 33, 1143–1148. [Google Scholar] [CrossRef]
- Parsons, S.; Flack, H.D.; Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Crystallogr. 2013, B69, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Gubaidullin, A.T.; Samigullina, A.I.; Bredikhina, Z.A.; Bredikhin, A.A. Crystal structure of chiral ortho-alkyl phenyl ethers of glycerol: True racemic compound, normal, false and anomalous conglomerates within the single five-membered family. CrystEngComm 2014, 16, 6716–6729. [Google Scholar] [CrossRef]
- Bredikhin, A.A.; Bredikhina, Z.A.; Gubaidullin, A.T. Chirality-dependent supramolecular synthons based on the 1,3-oxazolidin-2-one framework: Chiral drugs mephenoxalone, metaxalone and other 114 examples. CrystEngComm 2020, 22, 7252–7261. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Program for Empirical X-ray Absorption Correction; Bruker-Nonius: Delft, The Netherlands, 2004. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystalogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- BrukerAXS. APEX2 (Version 2.1), SAINTPlus. Data Reduction and Correction Program (Version 7.31A), Bruker Advanced X-ray Solutions; BrukerAXS: Madison, WI, USA, 2006. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bredikhin, A.A.; Bredikhina, Z.A.; Samigullina, A.I.; Gubaidullin, A.T. 3-(2-Chloro-5-methylphenoxy)propane-1,2-diol. Molbank 2023, 2023, M1624. https://doi.org/10.3390/M1624
Bredikhin AA, Bredikhina ZA, Samigullina AI, Gubaidullin AT. 3-(2-Chloro-5-methylphenoxy)propane-1,2-diol. Molbank. 2023; 2023(2):M1624. https://doi.org/10.3390/M1624
Chicago/Turabian StyleBredikhin, Alexander A., Zemfira A. Bredikhina, Aida I. Samigullina, and Aidar T. Gubaidullin. 2023. "3-(2-Chloro-5-methylphenoxy)propane-1,2-diol" Molbank 2023, no. 2: M1624. https://doi.org/10.3390/M1624
APA StyleBredikhin, A. A., Bredikhina, Z. A., Samigullina, A. I., & Gubaidullin, A. T. (2023). 3-(2-Chloro-5-methylphenoxy)propane-1,2-diol. Molbank, 2023(2), M1624. https://doi.org/10.3390/M1624








