(±)-N-(3-Chlorophenethyl)-2-(6-methoxynaphthalen-2-yl)propanamide
Abstract
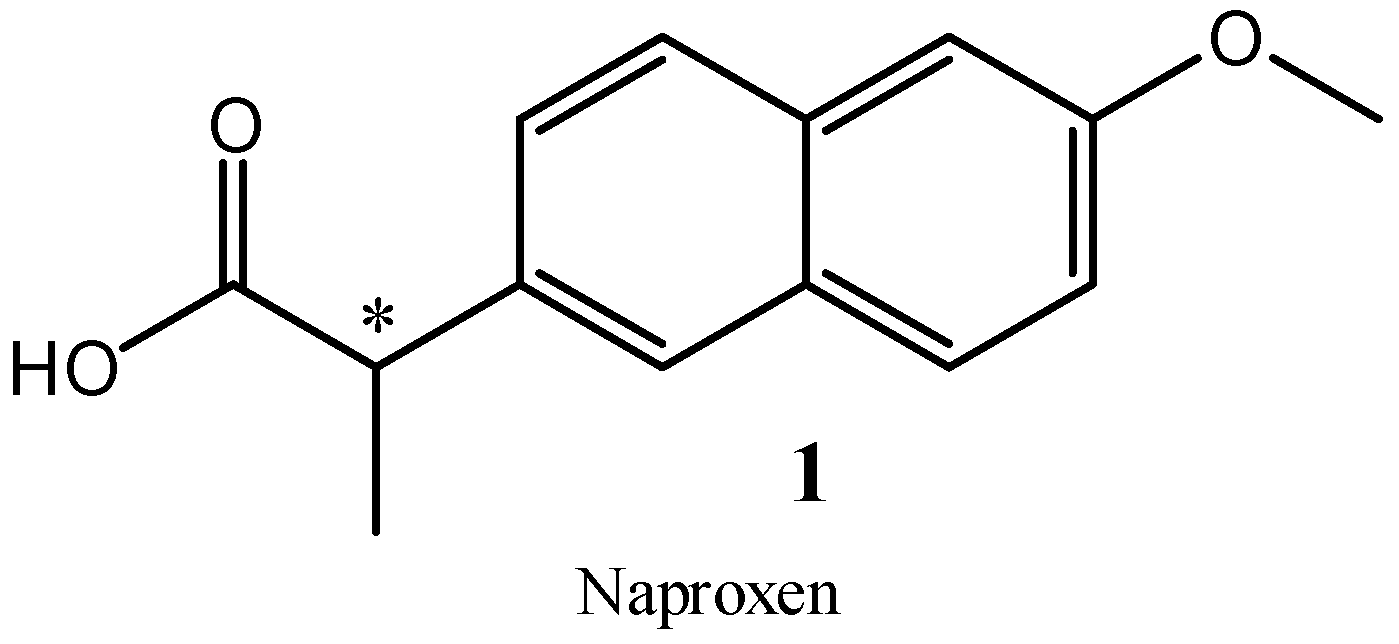
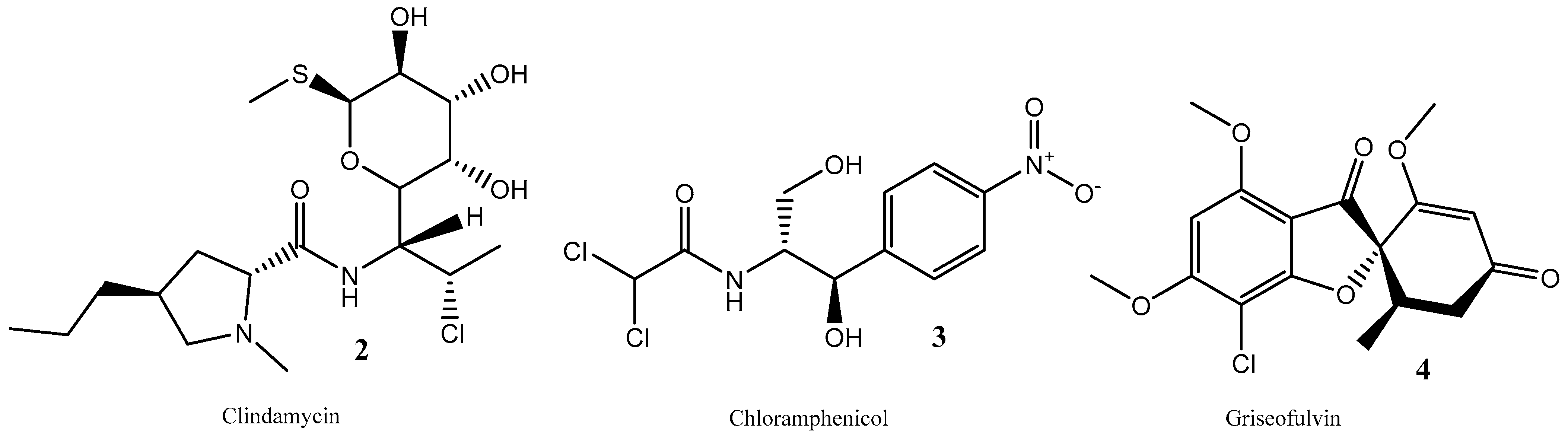
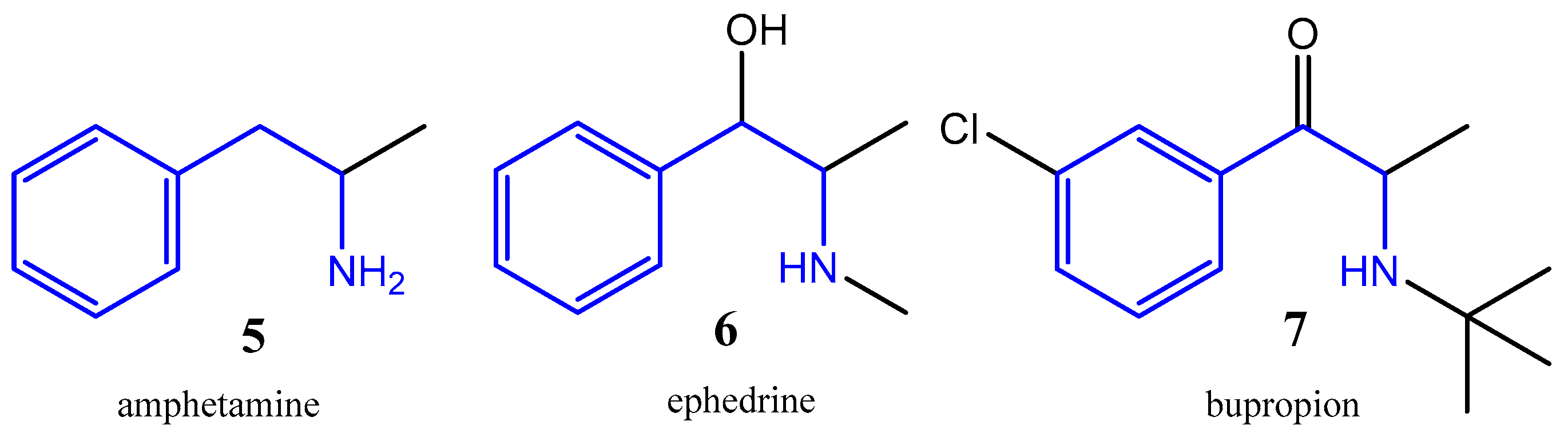
:1. Introduction
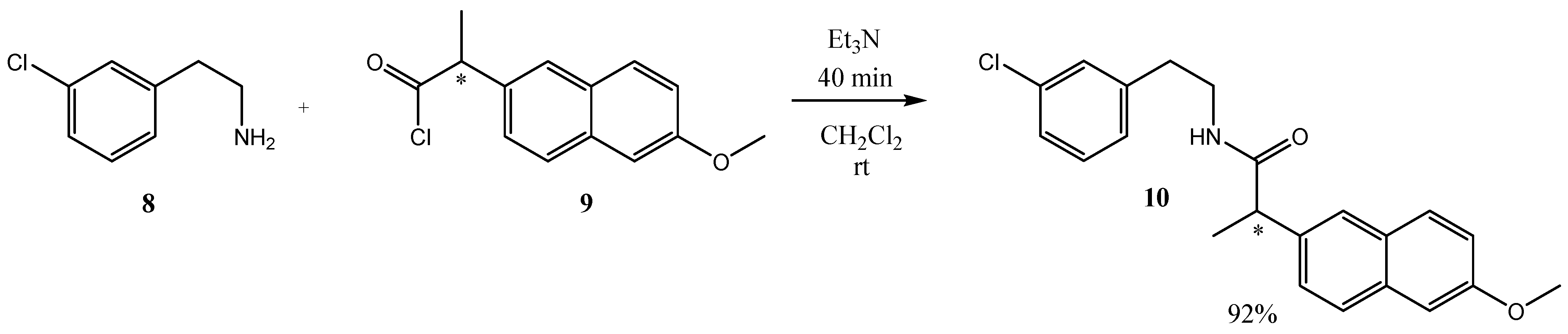
2. Results
3. Materials and Methods
3.1. (±)-2-(6-Methoxynaphthalen-2-yl)propanoyl Chloride 9
3.2. Synthesis of (±)-N-(3-Chlorophenethyl)-2-(6-methoxynaphthalen-2-yl)propanamide 10
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Black, J.G. Microbiology: Principles and Explorations, 8th ed.; Wiley: New York, NY, USA, 2012. [Google Scholar]
- Jachak, S. Cyclooxygenase inhibitory natural products: Current status. Curr. Med. Chem. 2006, 13, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Hileman, B. Concerns broaden over chlorine and chlorinated hydrocarbons. Chem. Eng. News 1993, 19, 11–20. [Google Scholar] [CrossRef]
- Henschler, D. Toxicity of chlorinated organic compounds: Effect of the introduction of chlorine in organic molecules. Angew. Chem. Int. Ed. Engl. 1994, 33, 1920–1935. [Google Scholar] [CrossRef]
- Naumann, K. Influence of chlorine substituents on biological activity of chemicals: A review. Pest Manag. Sci. 2000, 56, 3–21. [Google Scholar] [CrossRef]
- Fang, W.-Y.; Ravindar, L.; Rakesh, K.; Manukumar, H.; Shantharam, C.; Alharbi, N.; Qin, H.-L. Synthetic approaches and pharmaceutical applications of chloro-containing molecules for drug discovery: A critical review. Eur. J. Med. Chem. 2019, 173, 117–153. [Google Scholar] [CrossRef] [PubMed]
- Kosjek, T.; Heath, E. Halogenated heterocycles as pharmaceuticals. In Halogenated Heterocycles, 1st ed.; Iskra, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 27, pp. 219–246. [Google Scholar] [CrossRef]
- Berry, M. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J. Neurochem. 2004, 90, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Smith, T. Phenethylamine and related compounds in plants. Phytochemistry 1977, 16, 9–18. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manolov, S.; Ivanov, I.; Bojilov, D.; Nikolova, G. (±)-N-(3-Chlorophenethyl)-2-(6-methoxynaphthalen-2-yl)propanamide. Molbank 2023, 2023, M1625. https://doi.org/10.3390/M1625
Manolov S, Ivanov I, Bojilov D, Nikolova G. (±)-N-(3-Chlorophenethyl)-2-(6-methoxynaphthalen-2-yl)propanamide. Molbank. 2023; 2023(2):M1625. https://doi.org/10.3390/M1625
Chicago/Turabian StyleManolov, Stanimir, Iliyan Ivanov, Dimitar Bojilov, and Gabriela Nikolova. 2023. "(±)-N-(3-Chlorophenethyl)-2-(6-methoxynaphthalen-2-yl)propanamide" Molbank 2023, no. 2: M1625. https://doi.org/10.3390/M1625
APA StyleManolov, S., Ivanov, I., Bojilov, D., & Nikolova, G. (2023). (±)-N-(3-Chlorophenethyl)-2-(6-methoxynaphthalen-2-yl)propanamide. Molbank, 2023(2), M1625. https://doi.org/10.3390/M1625







