Regioselectivity Amination of Usnic Acid by Ammonia in Water
Abstract
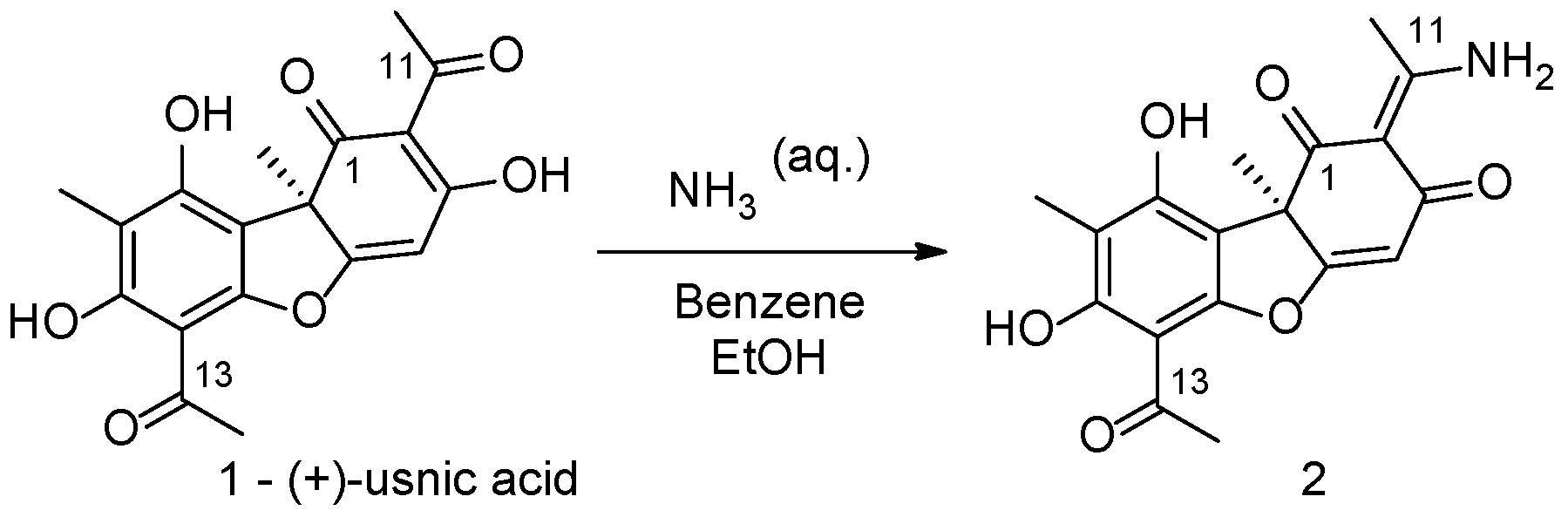
1. Introduction
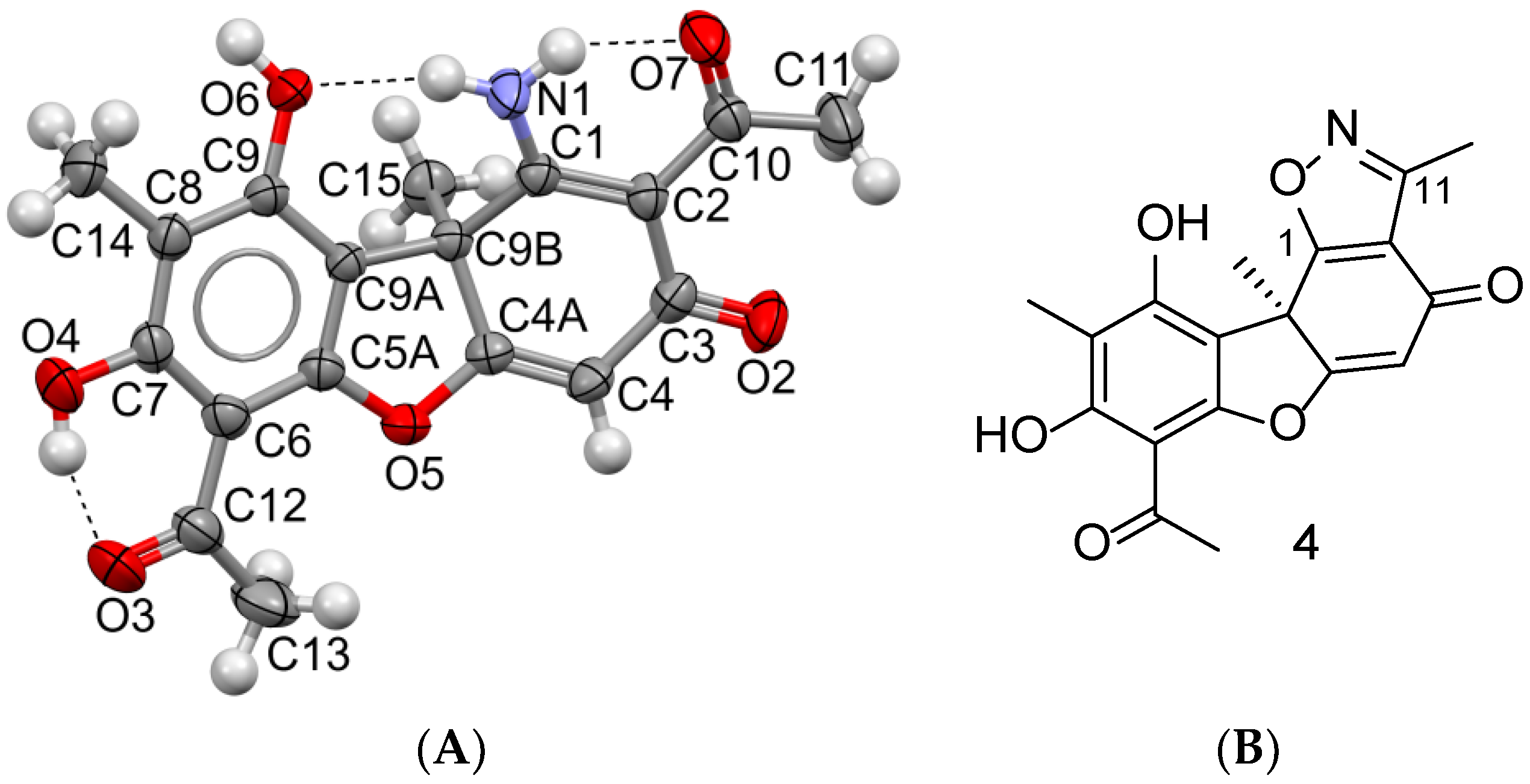
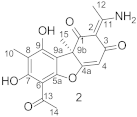
2. Results and Discussion
3. Materials and Methods
3.1. Procedure for the Usnic Acid C(1)-Amination Reaction
3.2. General Procedure for the Usnic Acid Amination Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macedo, D.C.S.; Almeida, F.J.F.; Wanderley, M.S.O.; Ferraz, M.S.; Santos, N.P.S.; López, A.M.Q.; Santos-Magalhães, N.S.; Lira-Nogueira, M.C.B. Usnic acid: From an ancient lichen derivative to promising biological and nanotechnology applications. Phytochem. Rev. 2021, 20, 609–630. [Google Scholar] [CrossRef]
- Wang, H.; Xuan, M.; Huang, C.; Wang, C. Advances in Research on Bioactivity, Toxicity, Metabolism, and Pharmacokinetics of Usnic Acid in vitro and in vivo. Molecules 2022, 27, 7469. [Google Scholar] [CrossRef] [PubMed]
- Luzina, O.A.; Salakhutdinov, N.F. Biological activity of usnic acid and its derivatives: Part 1. Activity against unicellular organisms. Russ. J. Bioorg. Chem. 2016, 42, 115–132. [Google Scholar] [CrossRef]
- Luzina, O.A.; Salakhutdinov, N.F. Biological activity of usnic acid and its derivatives: Part 2. Effects on higher organisms. Molecular and physicochemical aspects. Russ. J. Bioorg. Chem. 2016, 42, 249–268. [Google Scholar] [CrossRef]
- Luzina, O.A.; Salakhutdinov, N.F. Usnic acid and its derivatives for pharmaceutical use: A patent review (2000–2017). Expert Opin. Ther. Pat. 2018, 28, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, D.N.; Luzina, O.A.; Salakhutdinov, N.F. Usnic acid: Preparation, structure, properties and chemical transformations. Russ. Chem. Rev. 2012, 81, 747–768. [Google Scholar] [CrossRef]
- Borisov, S.A.; Luzina, O.A.; Khvostov, M.V.; Tolstikova, T.G.; Salakhutdinov, N.F. Synthesis and Pharmacological Evaluation of (+)-Usnic Acid Derivatives as Hypoglycemic Agents. Molbank 2022, 2022, M1459. [Google Scholar] [CrossRef]
- Kartsev, V.; Lichitsky, B.; Geronikaki, A.; Petrou, A.; Smiljkovic, M.; Kostic, M.; Radanovic, O.; Soković, M. Design, synthesis and antimicrobial activity of usnic acid derivatives. Med. Chem. Commun. 2018, 9, 870–882. [Google Scholar] [CrossRef]
- Bangalore, P.K.; Vagolu, S.K.; Bollikanda, R.K.; Veeragoni, D.K.; Choudante, P.C.; Misra, S.; Dharmarajan, S.; Balasubramanian, S.; Kantevari, S. Usnic Acid Enaminone-Coupled 1,2,3-Triazoles as Antibacterial and Antitubercular Agents. J. Nat. Prod. 2019, 83, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, V.M.; Nagi, R.V.; Kancharana, B.R.; Nishanth, J. Synthesis and antiproliferative activity of novel (+)- usnic acid analogues. J. Asian Nat. Prod. Res. 2020, 22, 562–577. [Google Scholar] [CrossRef]
- Bruno, M.; Trucchi, B.; Monti, D.; Romeo, S.; Kaiser, M.; Verotta, L. Synthesis of a Potent Antimalarial Agent through Natural Products Conjugation. ChemMedChem 2013, 8, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Kutney, J.P.; Sánchez, I.H. Studies in the usnic acid series. I. The condensation of (+)-usnic acid with aliphatic and aromatic amines. Can. J. Chem. 1976, 54, 2795–2803. [Google Scholar] [CrossRef]
- Yu, X.; Guo, Q.; Su, G.; Yang, A.; Hu, Z.; Qu, C.; Wan, Z.; Li, R.; Tu, P.; Chai, X. Usnic Acid Derivatives with Cytotoxic and Antifungal Activities from the Lichen Usnea longissima. J. Nat. Prod. 2016, 79, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.B.; Wang, J.; Saksena, A.K.; Girijavallabhan, V.; Ganguly, A.K.; Chan, T.-M.; McPhail, A.T. Synthesis of (+)-8-methyl cercosporamide: Stereochemical correlation of natural (−)-cercosporamide with (+)-usnic acid. Tetrahedron 1992, 48, 4757–4766. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta. Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta. Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]

| Ammonia Amount | Compound 2 | Compound 3 |
|---|---|---|
| 12.5 eq. | 72 | 28 |
| 62.5 eq. | 53 | 47 |
| 125 eq. | 34 | 66 |
| Temperature * | Compound 2 | Compound 3 |
|---|---|---|
| 9 °C | trace | 100 |
| 23 °C | 23 | 77 |
| 100 °C | 34 | 66 |
 |  | ||||
|---|---|---|---|---|---|
| Comparison of PMR | Comparison of 13C NMR | ||||
| Atom * | Signals of 2 | Signals of 3 | Atom * | Signals of 2 | Signals of 3 |
| H-4 | 5.79 | 5.75 | C-1 | 197.55 | 174.52 |
| CH3-10 | 1.93 | 2.02 | C-2 | 100.79 | 103.67 |
| CH3-12 | 2.51 | 2.42 | C-3 | 188.46 | 184.32 |
| CH3-14 | 2.59 | 2.63 | C-4 | 102.48 | 102.79 |
| CH3-15 | 1.59 | 1.72 | C-4a | 172.91 | 171.18 |
| OH-7 | 13.37 | 13.51 | C-9a | 105.05 | 107.11 |
| OH-9 | 12.26 | — | C-9b | 56.02 | 51.13 |
| NH2-1 | — | 9.41 and 11.35 | C-11 | 175.80 | 198.75 |
| NH2-11 | 9.82 and 11.53 | — | C-12 | 24.47 | 32.54 |
| C-15 | 30.94 | 33.80 | |||
| Mp (°C) | [α]D24.6 * (deg) | Ultraviolet Data * λmax (nm) and log10ε (in Parentless) | IR (cm−1) | |
|---|---|---|---|---|
| 2 | 271–273 | +638 | 227 (4.40), 293 (4.51) | 2500–3400, 1697, 1626, 1549 |
| 3 | 269–271 | +599 | 215 (4.33), 226 (4.33), 284 (4.43), 323 (4.08) | 2500–3600, 1700, 1624, 1579 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filimonov, A.; Luzina, O.; Gatilov, Y.; Salakhutdinov, N. Regioselectivity Amination of Usnic Acid by Ammonia in Water. Molbank 2023, 2023, M1618. https://doi.org/10.3390/M1618
Filimonov A, Luzina O, Gatilov Y, Salakhutdinov N. Regioselectivity Amination of Usnic Acid by Ammonia in Water. Molbank. 2023; 2023(2):M1618. https://doi.org/10.3390/M1618
Chicago/Turabian StyleFilimonov, Aleksandr, Olga Luzina, Yuri Gatilov, and Nariman Salakhutdinov. 2023. "Regioselectivity Amination of Usnic Acid by Ammonia in Water" Molbank 2023, no. 2: M1618. https://doi.org/10.3390/M1618
APA StyleFilimonov, A., Luzina, O., Gatilov, Y., & Salakhutdinov, N. (2023). Regioselectivity Amination of Usnic Acid by Ammonia in Water. Molbank, 2023(2), M1618. https://doi.org/10.3390/M1618






