Abstract
A novel free fatty acid receptor 1 (FFAR1) agonist has been synthesized and evaluated in vitro. The synthesis of the title compound was performed from commercially available 4-hydroxybenzaldehyde, 2-thiophenecarboxaldehyde, and (+)-camphor. The compound was shown to have an affinity to FFAR1.
1. Introduction
Type 2 diabetes (DM2) is a common endocrine disease characterized by sustained high blood sugar levels due to a pathological condition in which cells fail to normally respond to the hormone insulin [1]. Chronic hyperglycemia leads to the development of complications such as nephropathy [2], retinopathy [3], atherosclerosis [4], and cardiovascular disease [5].
Despite the large number of available treatments, none of them are universal, which, combined with their various side effects, makes the search for new therapies for DM2 an urgent task.
Free fatty acid receptor 1 (FFAR1), also known as GPR40, is considered one of the promising targets for DM2 therapy. Activation of this receptor leads to the normalization of blood glucose levels through a glucose-dependent increase in insulin secretion [6].
Numerous studies demonstrated that phenylpropanoic acid derivatives are effective agonists of this receptor [7]. Previously, based on the LY2881835 structure [8], we synthesized QS-528 in which the spirocyclic fragment was replaced with a bicyclic bornyl moiety derived from camphor (Figure 1). QS-528 has been shown to exhibit an affinity to FFAR1 during in vitro tests in the submicromolar concentration and also had a hypoglycemic effect in an oral glucose tolerance test (OGTT) on mice [9]. Further studies of this compound revealed other useful effects, such as reduction of fatty degeneration of the liver and a hepatoprotective effect in mice [10,11]. Based on our results, we consider the structural analogues of QS-528 to be promising compounds for the therapy of DM2.
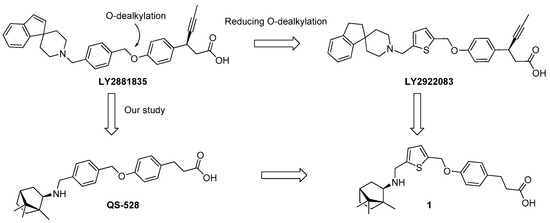
Figure 1.
FFAR1 agonists.
Some papers [12] note that the replacement of the benzene fragment with the thiophene fragment in the agonist structure may lead to greater metabolic stability due to a decrease in the degree of O-dealkylation (Figure 1).
The aim of this work is to synthesize compound 1, which is a structural analogue of compound QS-528, in which the linker between phenylpropanoic acid and the terpene backbone will be a thiophene ring rather than a benzene ring, and to evaluate its affinity for the FFA1 receptor.
2. Results
2.1. Chemistry
4-(Chloromethyl)thiophene-2-carbaldehyde 2 was reacted with a small excess of methyl 3-(4-hydroxyphenyl)propanoate 3 in N,N-dimethylformamide (DMF) in the presence of an excess of potassium carbonate as a base at room temperature. After 4 days, no substance 2 was observed in the reaction mixture. After the treatment of the reaction mixture and subsequent chromatography, aldehyde 4 was isolated in 64% yield. Reductive amination of aldehyde 4 with a small excess of (R)-(−)-isobornylamine resulted in the formation of compound 5 in 87% yield after chromatography. Treatment with lithium hydroxide of compound 5 in a mixture of tetrahydrofuran (THF)–methanol–water upon cooling resulted in the hydrolysis of the ester group, and after the treatment of the reaction mixture with a dilute hydrochloric acid solution, the target compound 1 was isolated as a hydrochloride salt in 52% yield (Scheme 1).
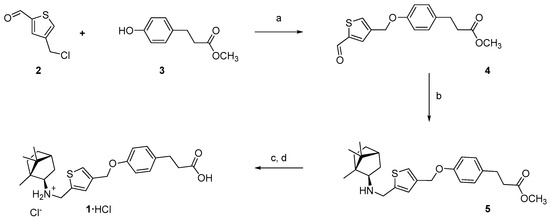
Scheme 1.
Synthesis of compound 1 as HCl salt. Reagents and conditions: (a) K2CO3, DMF, 64%; (b) (R)-(−)-isobornylamine, NaBH(OAc)3, AcOH, CH2Cl2, 87%; (c) LiOH, MeOH, THF, H2O; (d) 2 M HCl, H2O, 52%.
2.2. In Vitro FFAR1 Binding Evaluation
Compound 1 and QS-528 were tested for FFAR1 activation using a Cayman FFAR1 Assay Kit in a single concentration (10 μM). The results obtained are shown in Figure 2. Partial agonist GW9508 was used as a positive control at the same concentration. For compound QS-528, we reproduced the results previously obtained on another test system [9]. QS-528 efficacy against GW9508 was approximately 108% just as in the previously published work. Compound 1 not only exhibited activity at a similar concentration but also showed similar efficacy to compound QS-528.
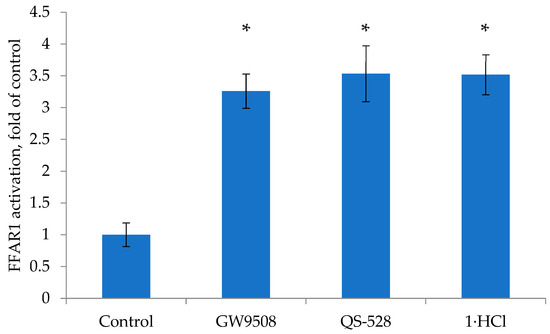
Figure 2.
Effects of compound 1 and QS-528 (10 μM) on FFAR1 activation. Results are mean ± SD. * p < 0.05 as compared with control.
3. Materials and Methods
3.1. General
Dichloromethane was refluxed over phosphorus pentoxide and distilled. Tetrahydrofuran (THF) was refluxed over sodium/benzophenone and distilled in inert atmosphere. N,N-dimethylformamide (DMF) was stirred over calcium hydride (5% w/v) overnight, filtered, and distilled in vacuum. 4-Hydroxybenzaldehyde and thiophene-2-carbaldehyde were obtained from Acros Organics. (+)-Camphor was purchased from TCI Co. (Tokyo, Japan). 1H and 13C NMR spectra were acquired on Bruker spectrometers AV-400 at 400.13 MHz (1H) and DRX-500 at 500.13 MHz (1H) and 125.76 MHz (13C) in deuterated chloroform (CDCl3) or deuterated dimethyl sulfoxide (DMSO-d6); chemical shifts in parts per million (ppm) were measured relative to residual CHCl3 ((CHCl3) 7.26, (CHCl3) 77.00 ppm) or DMSO-d6 ((DMSO-d6) 2.50, (DMSO-d6) 39.51 ppm), and J was measured in hertz. The attached proton test (APT) modification of the J-modulated spin-echo (JMOD) experiment was performed. The structure of the products was determined by means of 1H and 13C NMR spectra (Figures S1–S6). High-resolution mass spectrometry (HRMS) was conducted using a DFS Thermo Scientific spectrometer in full scan mode (m/z 15–500, 70 eV electron impact ionization, and direct sample injection). Column chromatography was performed on silica gel (60–200 mesh, Macherey-Nagel). Thin-layer chromatography (TLC) was performed on Merck TLC Silica gel 60 F254.
3.2. Synthesis
4-(Chloromethyl)thiophene-2-carbaldehyde 2 was synthesized according to the procedure described from thiophene-2-carbaldehyde [13].
Methyl 3-(4-hydroxyphenyl)propanoate 3 was synthesized from 4-hydroxybenzaldehyde according to the procedure described [9].
(1R,2R,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-amine ((R)-(−)-isobornylamine) was synthesized from (R)-(+)-camphor according to the procedure described [9].
- Methyl 3-(4-((5-formylthiophen-3-yl)methoxy)phenyl)propanoate 4. A solution of chloride (0.77 g, 4.8 mmol), phenol (1.21 g, 6.7 mmol), and K2CO3 (1.99 g, 14.4 mmol) in DMF (10 mL) was stirred at room temperature for 4 days. A solution was poured into water (50 mL) and extracted with ethyl acetate (3 × 30 mL). Combined organic extracts were washed with water (30 mL) and brine (30 mL) and dried over anhydrous magnesium sulfate overnight. The drying agent was filtered off and washed with a small portion of ethyl acetate. Combined solutions were evaporated under reduced pressure, and the residue was purified over silica gel column chromatography using chloroform as an eluent to obtain 934 mg (yield 64%) of compound 4 as a slightly yellow oil. Rf 0.33 (Hexane-EtOAc, 5:4). 1H NMR (400 MHz, CDCl3): 9.91 (d, J = 1.3 Hz, 1H, CHO), 7.80 (d, J = 1.3 Hz, 1H, Th-5-H), 7.72 (s, 1H, Th-3-H), 7.16–7.11 (m, 2H, Ph-2,6-H), 6.90–6.86 (m, 2H, Ph-3,5-H), 5.05 (s, 2H, OCH2), 3.66 (s, 3H, OCH3), 2.90 (t, J = 7.7 Hz, 2H, CH2CH2COOMe), 2.60 (t, J = 7.7 Hz, 2H, CH2CH2COOMe). 13C NMR (126 MHz, CDCl3): 182.9 (CHO), 173.3 (COOMe), 156.6 (Ph-4), 144.4 (Th-2), 139.5 (Th-4), 135.7 (Th-5), 133.3 (Ph-1), 132.2 (Th-3), 129.4 (2C, Ph-2,6), 114.7 (2C, Ph-3,5), 65.0 (CH2O), 51.6 (OCH3), 35.8 (CH2CH2COOMe), 30.0 (CH2CH2COOMe). HRMS for C16H16O432S+ calcd 304.0764, found 304.0762 [M]+.
- Methyl 3-(4-((5-((((1R,2R,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-yl)amino)methyl)thio- phen-3-yl)methoxy)phenyl)propanoate 5. To an ice-cooled stirred solution of aldehyde 4 (280 mg, 0.92 mmol), isobornylamine (162 mg, 1.06 mmol), and acetic acid (0.1 mL) in dichloromethane (7 mL), sodium triacetoxyborohydride (390 mg, 1.84 mmol) was added portion-wise in 5 min; then, after 30 min cooling, it was removed, and the solution was stirred overnight at room temperature. Dichloromethane (20 mL) and 5% potassium carbonate (10 mL) solution were added. The organic layer was separated, and the water layer was extracted with dichloromethane (2 × 10 mL). Combined organic layers were washed with 5% potassium carbonate (10 mL) solution, water (10 mL), and brine (10 mL) and dried over magnesium sulfate. The drying agent was filtered off and washed with a small portion of dichloromethane. Combined solutions were evaporated under reduced pressure, and the residue was purified over silica gel column chromatography using chloroform–methanol 100:1 as an eluent to obtain 353 mg (yield 87%) of compound 5 as a slightly yellow solid. Rf 0.69 (CHCl3-EtOH, 9:1). M.p. 72–74 °C. 1H NMR (500 MHz, CDCl3): δ = 7.15 (s, 1H, Th-2-H), 7.12 (d, J = 8.5 Hz, 2H, Ph-2,6-H), 6.94 (s, 1 H, Th-4-H), 6.89 (d, J = 8.5 Hz, 2H, Ph-3,5-H), 4.95 (s, 2H, CH2Cl), 3.93–3.88 (m, 1H, CH2NH), 3.85–3.80 (m, 1H, CH2NH), 3.67 (s, 3H, OCH3), 2.90 (t, J = 7.8 Hz, 2H, CH2CH2COOMe), 2.64–2.58 (m, 3H CH2CH2COOMe, Bornyl-2-Hendo), 1.73–1.47 (m, 5H, Bornyl-3-Hexo, Bornyl-3-Hendo, Bornyl-4-H, Bornyl-5-Hexo, Bornyl-6-Hexo), 1.30 (br. s., 1H, CH2NH), 1.11–1.03 (m, 5H, Bornyl-5-Hendo, Bornyl-6-Hendo, CH3), 0.89 (s, 3H, CH3), 0.82 (s, 3H, CH3). 13C NMR (126 MHz, CDCl3): δ = 173.4 (COOMe), 157.1 (Ph-4), 146.9 (Th-5-C), 137.2 (Th-3-C), 132.8 (Ph-1-C), 129.2 (2C, Ph-2,6-C), 124.0 (Th-4-C), 121.9 (Th-2-C), 114.7 (2C, Ph-3,5-C), 65.8 (Bornyl-2-C), 65.8 (CH2O), 51.6 (OCH3), 48.4 (Bornyl-1-C), 47.2 (CH2NH), 46.8 (Bornyl-7-C), 45.2 (Bornyl-4-C), 38.7 (Bornyl-3-C), 36.7 (Bornyl-6-C), 35.9 (CH2CH2COOMe), 30.0 (CH2CH2COOMe), 27.3 (Bornyl-5-C), 20.6 (CH3), 20.5 (CH3), 12.1 (CH3). HRMS for C26H35O3N32S+ calcd 441.2332, found 441.2335 [M]+.
- (1R,2R,4R)-N-((4-((4-(2-Carboxyethyl)phenoxy)methyl)thiophen-2-yl)methyl)-1,7,7-trimethyl-biyclo[2.2.1]heptan-2-aminium chloride 1·HCl. Methyl ester 5 (166 mg, 0.38 mmol) was dissolved in a mixture of tetrahydrofuran (1.5 mL) and methanol (0.5 mL), and then 1 mL of water was added. To the obtained ice-cooled stirred solution, lithium hydroxide monohydrate (62 mg, 1.48 mmol) was added. After the completion of the reaction (controlled by TLC), tetrahydrofuran and methanol were evaporated under reduced pressure. Water (10 mL) was added to a remaining solution, then 2 M hydrochloric acid was added drop-wise until pH 2–3, and the obtained solution was placed in a fridge overnight. The precipitate was filtered, washed with cold water, and dried in a vacuum desiccator over phosphorus pentoxide to obtain 92 mg (yield 52%) of compound 1 as a white solid. Rf 0.11 (CHCl3-EtOH-Et3N, 5:1:0.05), as 1·Et3N salt. M.p. (with decomposition) 167–170 °C. 1H NMR (400 MHz, DMSO-d6): δ = 12.08 (br. s, 1H, COOH), 8.65–8.85 (m, 2H, CH2N+H2), 7.64 (s, 1H, Th-5-H), 7.46 (s, 1H, Th-3-H), 7.12 (d, J = 8.6, 2H, Ph-2,6-H), 6.89 (d, J = 8.6, 2H, Ph-3,5-H), 5.03 (s, 2H, CH2O), 4.28–4.40 (m, 2H, CH2N+H2), 2.99 (m, 1H, Bornyl-2-Hendo), 2.70–2.77 (t, J = 7.5, 2H, CH2CH2COOH), 2.44–2.48 (t, J = 7.5, 2H, CH2CH2COOH), 2.04–2.13 (m, 1H, Bornyl-3-Hexo), 1.43–1.75 (m, 4H, Bornyl-3-Hendo, Bornyl-4-H, Bornyl-5-Hexo, Bornyl-6-Hexo), 0.96–1.06 (m, 2H, Bornyl-5-Hendo, Bornyl-6-Hendo), 0.92 (s, 3H, CH3), 0.87 (s, 3H, CH3), 0.78 (s, 3H, CH3). 13C NMR (126 MHz, DMSO-d6): δ = 173.9 (COOH), 156.4 (Ph-4-C), 138.1 (Th-3-C), 133.2 (Th-5-C), 133.1 (Ph-1-C), 131.9 (Th-4-C), 129.2 (C2, Ph-2,6-C), 126.5 (Th-2-C), 114.6 (C2, Ph-3,5-C), 64.7 (CH2O), 63.9 (Bornyl-2-C), 48.5 (CH2N+H2), 46.9 (Bornyl-1-C), 44.8 (Bornyl-7-C), 44.2 (Bornyl-4-C), 36.2 (Bornyl-3-C), 35.6 (CH2CH2COOH), 34.6 (Bornyl-6-C), 29.5 (CH2CH2COOH), 26.2 (Bornyl-5-C), 20.3 (CH3), 19.9 (CH3), 11.6 (CH3). HRMS for C25H33O3N32S+ calcd 427.2181, found 427.2184 [M]+.
3.3. FFAR1 Assay Kit
To determine the FFAR1 activation of compounds 1 and QS-528 «FFAR1 (GPR40) Reporter Assay Kit (Cayman Chemical)» was used. A known FFAR1 agonist, GW9508, as part of a kit, was used as a positive control. The assay kit was based on a transfection complex containing DNA constructs for FFAR1, an engineered G protein that directs Gαq activation signals to the Gαs pathway, and a cAMP response element-regulated secreted alkaline phosphatase (SEAP) reporter. HEK293T cells were seeded at a density of 5 × 104 cells/well into a 96-well plate coated with a transfection complex in DMEM (Servicebio) containing 10% FBS (Sigma-Aldrich). After culturing for 24 h, the medium was replaced by serum-free Opti-MEM (Gibco) with test compounds at concentrations of 10 μM. After 24 h, part of the supernatant was collected for assay of SEAP activity, which was measured by adding a luminescence-based substrate. Luminescence was measured using ClarioStar (BMG Labtech). FFAR1 activation was calculated relative to baseline luminescence (control group, without compounds).
4. Conclusions
We developed a method to synthesize 3-(4-((5-((((1R,2R,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-yl)amino)methyl)thiophen-2-yl)methoxy)phenyl)propanoic acid as a hydrochloride salt from commercially available 4-hydroxybenzaldehyde, thiophene-2-carbaldehyde, and (R)-(+)-camphor. The synthesized compound at a concentration of 10 μM was shown to have an affinity toward FFAR1 comparable with known agonists GW9508 and QS-528. Compound 1 is expected to have better metabolic stability than compound QS-528, making it a more promising compound.
Supplementary Materials
The following supporting information can be downloaded online: Figure S1. 1H NMR spectrum of methyl 3-(4-((5-formylthiophen-3-yl)methoxy)phenyl)propanoate 4. Figure S2. 13C NMR spectrum of methyl 3-(4-((5-formylthiophen-3-yl)methoxy)phenyl)propanoate 4. Figure S3. 1H NMR spectrum of methyl 3 (4-((5-(((1R,2R,4R)-1,7,7 trimethylbicyclo[2.2.1]heptan-2-ylamino)methyl)thiophen 3-yl)methoxy)phenyl)propanoate 5. Figure S4. 13C NMR spectrum of methyl 3 (4-((5-(((1R,2R,4R)-1,7,7 trimethylbicyclo[2.2.1]heptan-2-ylamino)methyl)thiophen 3-yl)methoxy)phenyl)propanoate 5. Figure S5. 1H NMR spectrum of (1R,2R,4R)-N-((4-((4-(2-carboxyethyl)phenoxy)methyl)thiophen-2-yl)methyl)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-aminium chloride 1•HCl. Figure S6. 13C NMR spectrum of (1R,2R,4R)-N-((4-((4-(2-carboxyethyl)phenoxy)methyl)thiophen-2-yl)methyl)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-aminium chloride 1•HCl.
Author Contributions
Conceptualization, S.O.K. and O.A.L.; methodology, S.O.K., O.A.L. and M.V.K.; investigation, S.O.K. and M.K.M.; data curation, O.A.L. and M.V.K.; writing—original draft preparation, S.O.K., M.K.M., O.A.L. and M.V.K.; writing—review and editing, O.A.L. and M.V.K.; supervision, T.G.T. and N.F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Russian Science Foundation grant № 21-73-00246.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge the Multi-Access Chemical Research Center SB RAS for spectral and analytical measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.K. Diabetic nephropathy—Complications and treatment. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Nentwich, M.M.; Ulbig, M.W. Diabetic retinopathy—Ocular complications of diabetes mellitus. World J. Diabetes 2015, 6, 489. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; Bornfeldt, K.E. Diabetes and atherosclerosis: Is there a role for hyperglycemia? J. Lipid Res. 2009, 50, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Poitout, V.; Lin, D.C. Modulating GPR40: Therapeutic promise and potential in diabetes. Drug Discov. Today 2013, 18, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Kuranov, S.O.; Luzina, O.A.; Salakhutdinov, N.F. FFA1 (GPR40) Receptor Agonists Based on Phenylpropanoic Acid as Hypoglycemic Agents: Structure–Activity Relationship. Russ. J. Bioorg. Chem. 2020, 46, 972–988. [Google Scholar] [CrossRef]
- Chen, Y.; Song, M.; Riley, J.P.; Hu, C.C.; Peng, X.; Scheuner, D.; Bokvist, K.; Maiti, P.; Kahl, S.D.; Montrose-Rafizadeh, C.; et al. A selective GPR40 (FFAR1) agonist LY2881835 provides immediate and durable glucose control in rodent models of type 2 diabetes. Pharmacol. Res. Perspect. 2016, 21, 278. [Google Scholar] [CrossRef] [PubMed]
- Kuranov, S.O.; Luzina, O.A.; Onopchenko, O.; Pishel, I.; Zozulya, S.; Gureev, M.; Salakhutdinov, N.F.; Krasavin, M. Exploring bulky natural and natural-like periphery in the design of p-(benzyloxy)phenylpropionic acid agonists of free fatty acid receptor 1 (GPR40). Bioorg. Chem. 2020, 99, 103830. [Google Scholar] [CrossRef] [PubMed]
- Kuranov, S.; Luzina, O.; Khvostov, M.; Baev, D.; Kuznetsova, D.; Zhukova, N.; Vassiliev, P.; Kochetkov, A.; Tolstikova, T.; Salakhutdinov, N. Bornyl Derivatives of p-(Benzyloxy)Phenylpropionic Acid: In Vivo Evaluation of Antidiabetic Activity. Pharmaceuticals 2020, 13, 404. [Google Scholar] [CrossRef] [PubMed]
- Pon`kina, D.; Kuranov, S.; Khvostov, M.; Zhukova, N.; Meshkova, Y.; Marenina, M.; Luzina, O.; Tolstikova, T.; Salakhutdinov, N. Hepatoprotective Effect of a New FFAR1 Agonist—N-Alkylated Isobornylamine. Molecules 2023, 28, 396. [Google Scholar] [CrossRef] [PubMed]
- Hamdouchi, C.; Kahl, S.D.; Lewis, A.P.; Cardona, G.R.; Zink, R.W.; Chen, K.; Eessalu, T.E.; Ficorilli, J.V.; Marcelo, M.C.; Otto, K.A.; et al. The Discovery, Preclinical, and Early Clinical Development of Potent and Selective GPR40 Agonists for the Treatment of Type 2 Diabetes Mellitus (LY2881835, LY2922083, and LY2922470). J. Med. Chem. 2016, 59, 10891–10916. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, Y.L.; Karmanova, I.B.; Volkenshtein, Y.B.; Belenkii, L.I. Reactions of aromatic and heteroaromatic compounds containing electron-acceptor substituents. Chem. Heterocycl. Compd. 1978, 14, 1196–1198. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).