Dibenzyl-(1S*,2S*)-2,3-dihydro-1H-indene-1,2-dicarboxylate
Abstract
1. Introduction
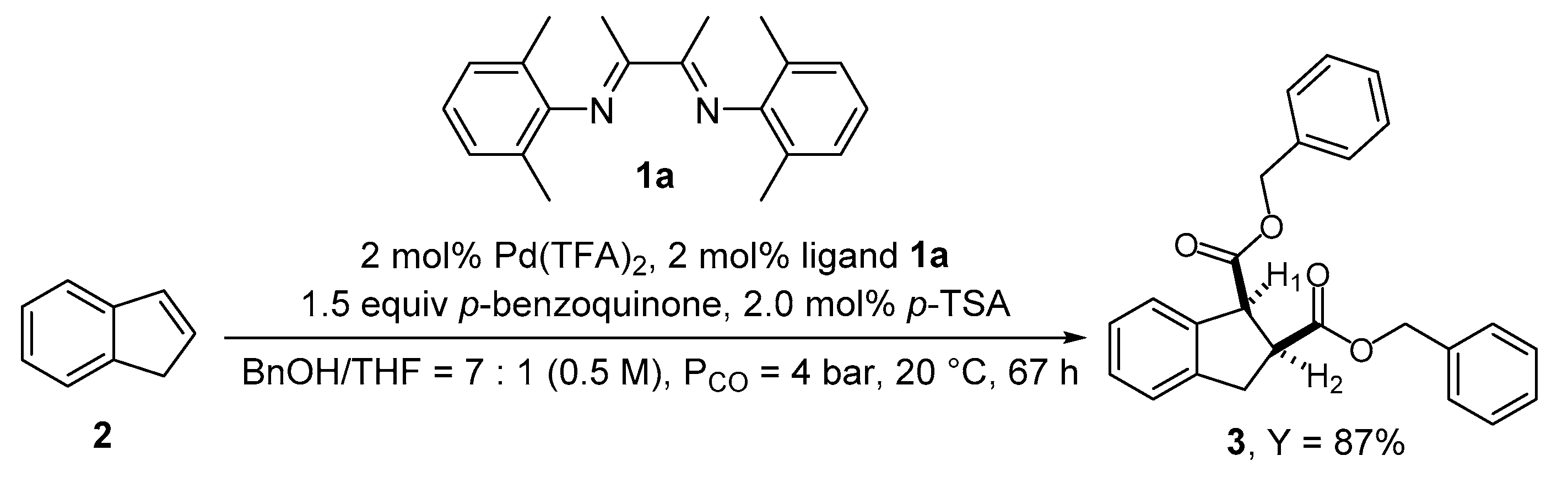
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, J.-B.; Geng, H.-Q.; Wu, X.-F. The Chemistry of CO: Carbonylation. Chem 2019, 5, 526–552. [Google Scholar] [CrossRef]
- Beller, M. Catalytic Carbonylation Reactions; Springer: Berlin, Germany, 2006. [Google Scholar] [CrossRef]
- Kollár, L. Modern Carbonylation Methods; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar] [CrossRef]
- Godard, C.; Muñoz, B.K.; Ruiz, A.; Claver, C. Pd-catalysed asymmetric mono- and bis-alkoxycarbonylation of vinylarenes. Dalton Trans. 2008, 8, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F.; Neumann, H.; Beller, M. Palladium-Catalyzed Oxidative Carbonylation Reactions. ChemSusChem 2013, 6, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-B. Recent Advances in Carbonylative Difunctionalization of Alkenes. Adv. Synth. Catal. 2020, 362, 3059–3080. [Google Scholar] [CrossRef]
- Nghiem, N.P.; Kleff, S.; Schwegmann, S. Succinic Acid: Technology Development and Commercialization. Fermentation 2017, 3, 26. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Dodd, P.; Leask, R.; Maric, M.; Cooper, D.G. Designing green plasticizers: Influence of alkyl chain length on biodegradation and plasticization properties of succinate based plasticizers. Chemosphere 2013, 91, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Heng, K.Y.; Kei, T.Y.; Kochhar, J.S.; Li, H.; Poh, A.-L.; Kang, L. Handbook of Cosmeceutical Excipients and Their Safeties; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Saxena, R.K.; Saran, S.; Isar, J.; Kaushik, R. Production and Applications of Succinic Acid. In Current Developments in Biotechnology and Bioengineering. Production, Isolation and Purification of Industrial Products; Pandey, A., Negi, S., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 601–630. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.-H.; Yi, H.; Lei, A. An Update on Oxidative C−H Carbonylation with CO. ACS Catal. 2022, 12, 7470–7485. [Google Scholar] [CrossRef]
- Aratani, T.; Tahara, K.; Takeuchi, S.; Kitamura, S.; Murai, M.; Fujinami, S.; Inomata, K.; Ukaji, Y. Asymmetric Bis(alkoxycarbonylation) Reaction of Cyclic Olefins Catalyzed by Palladium in the Presence of Copper(I) Triflate. Bull. Chem. Soc. Jpn. 2012, 85, 1225–1232. [Google Scholar] [CrossRef]
- Cho, Y.J.; Lim, Y.N.; Yoon, W.; Yun, H.; Jang, H.-Y. Palladium–Bis(carbene) Catalysts for the Bisalkoxycarbonylation of Olefins to Succinic Diesters. Eur. J. Org. Chem. 2017, 1139–1142. [Google Scholar] [CrossRef]
- Fini, F.; Beltrani, M.; Mancuso, R.; Gabriele, B.; Carfagna, C. Selective Aryl a-Diimine/Palladium-Catalyzed Bis-Alkoxycarbonylation of Olefins for the Synthesis of Substituted Succinic Diesters. Adv. Synth. Catal. 2015, 357, 177–184. [Google Scholar] [CrossRef]
- Olivieri, D.; Fini, F.; Mazzoni, R.; Zacchini, S.; Della Ca’, N.; Spadoni, G.; Gabriele, B.; Mancuso, R.; Zanotti, V.; Carfagna, C. Diastereospecific Bis-Alkoxycarbonylation of 1,2-disubstituted Olefins Catalyzed by Aryl α-Diimine Palladium(II) Catalysts. Adv. Synth. Catal. 2018, 360, 3507–3517. [Google Scholar] [CrossRef]
- Olivieri, D.; Tarroni, R.; Della Ca’, N.; Mancuso, R.; Gabriele, B.; Spadoni, G.; Carfagna, C. Bis-Alkoxycarbonylation of Acrylic Esters and Amides for the Synthesis of 2-Alkoxycarbonyl or 2-Carbamoyl Succinates. Adv. Synth. Catal. 2020, 362, 533–544. [Google Scholar] [CrossRef]
- Olivieri, D.; Tarroni, R.; Della Ca’, N.; Mancuso, R.; Gabriele, B.; Spadoni, G.; Carfagna, C. Combined Effect of Palladium Catalyst and the Alcohol to Promote the Uncommon Bis-Alkoxycarbonylation of Allylic Substrates. ChemCatChem 2022, 14, e202101923. [Google Scholar] [CrossRef]
- Olivieri, D.; Tarroni, R.; Carfagna, C. Bis(2-hydroxyethyl) 2-phenylsuccinate. MolBank 2022, 2022, M1456. [Google Scholar] [CrossRef]
- Mealli, C.; Manca, G.; Tarroni, R.; Olivieri, D.; Carfagna, C. Computational Overview of a Pd-Catalyzed Olefin Bis-alkoxycarbonylation Process. Organometallics 2020, 39, 1059–1069. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.; Brookhart, M. Late-Metal Catalysts for Ethylene Homo- and Copolymerization. Chem. Rev. 2000, 100, 1169–1204. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivieri, D.; Tarroni, R.; Carfagna, C. Dibenzyl-(1S*,2S*)-2,3-dihydro-1H-indene-1,2-dicarboxylate. Molbank 2023, 2023, M1586. https://doi.org/10.3390/M1586
Olivieri D, Tarroni R, Carfagna C. Dibenzyl-(1S*,2S*)-2,3-dihydro-1H-indene-1,2-dicarboxylate. Molbank. 2023; 2023(1):M1586. https://doi.org/10.3390/M1586
Chicago/Turabian StyleOlivieri, Diego, Riccardo Tarroni, and Carla Carfagna. 2023. "Dibenzyl-(1S*,2S*)-2,3-dihydro-1H-indene-1,2-dicarboxylate" Molbank 2023, no. 1: M1586. https://doi.org/10.3390/M1586
APA StyleOlivieri, D., Tarroni, R., & Carfagna, C. (2023). Dibenzyl-(1S*,2S*)-2,3-dihydro-1H-indene-1,2-dicarboxylate. Molbank, 2023(1), M1586. https://doi.org/10.3390/M1586






