Ethyl 12-Sulfamoyl-abieta-8,11,13-trien-18-oate
Abstract
1. Introduction
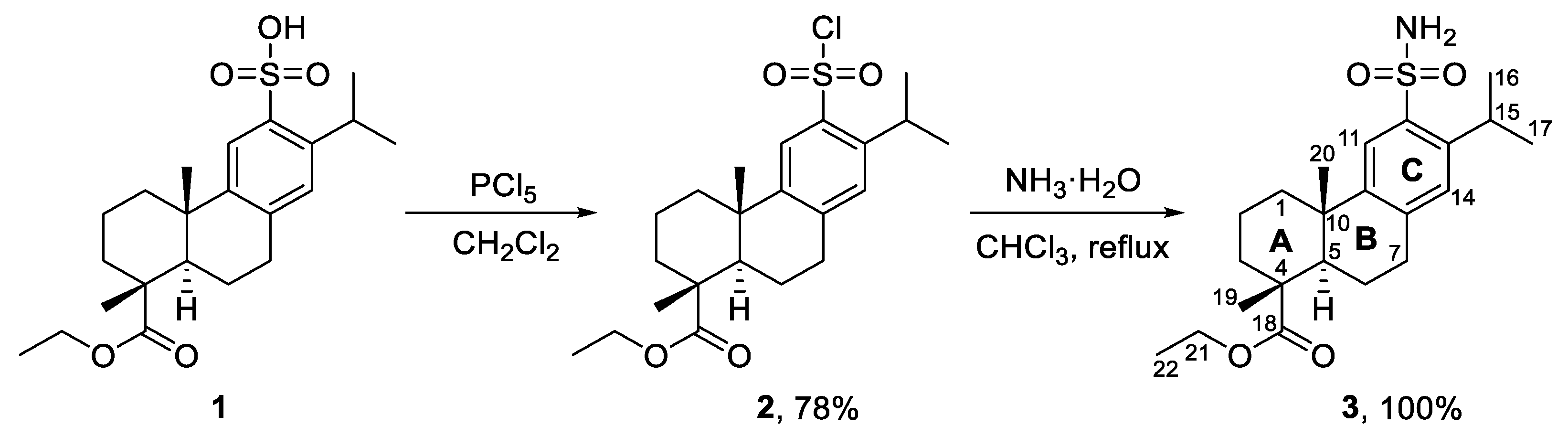
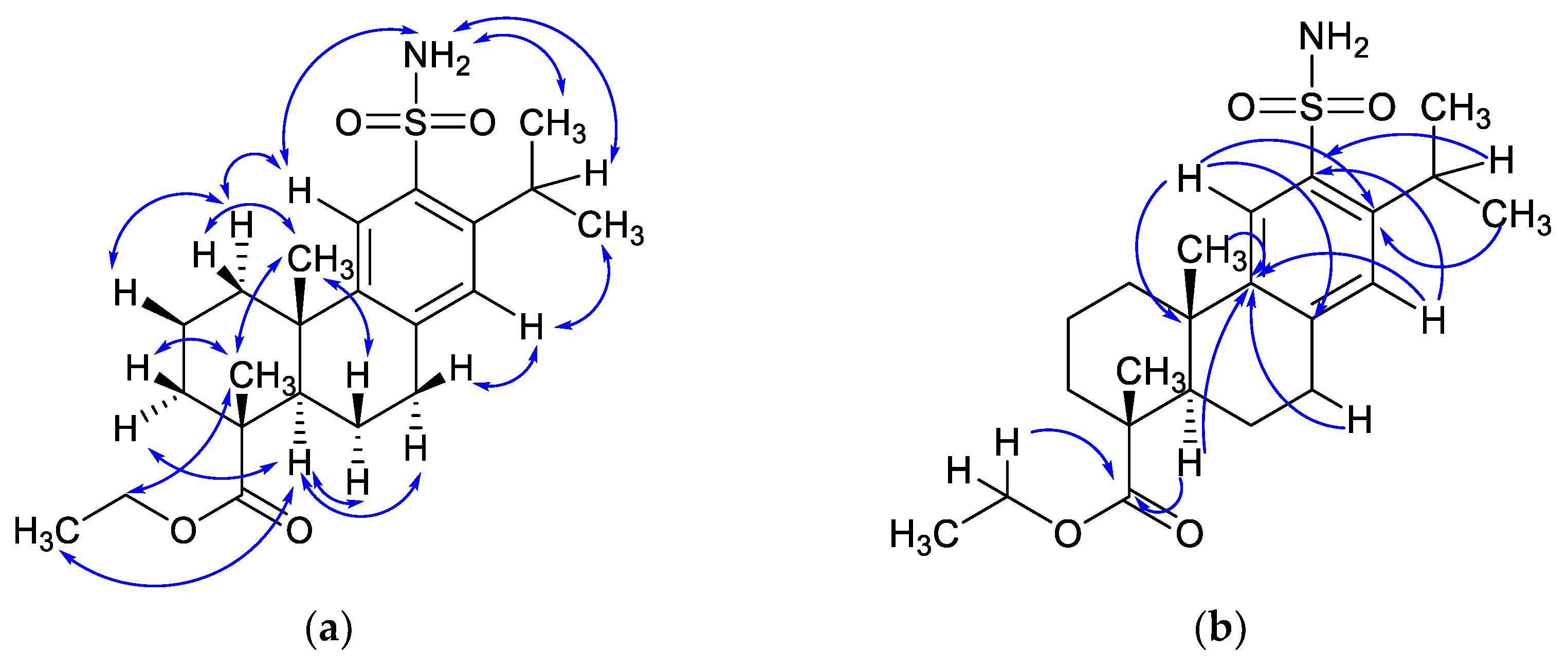
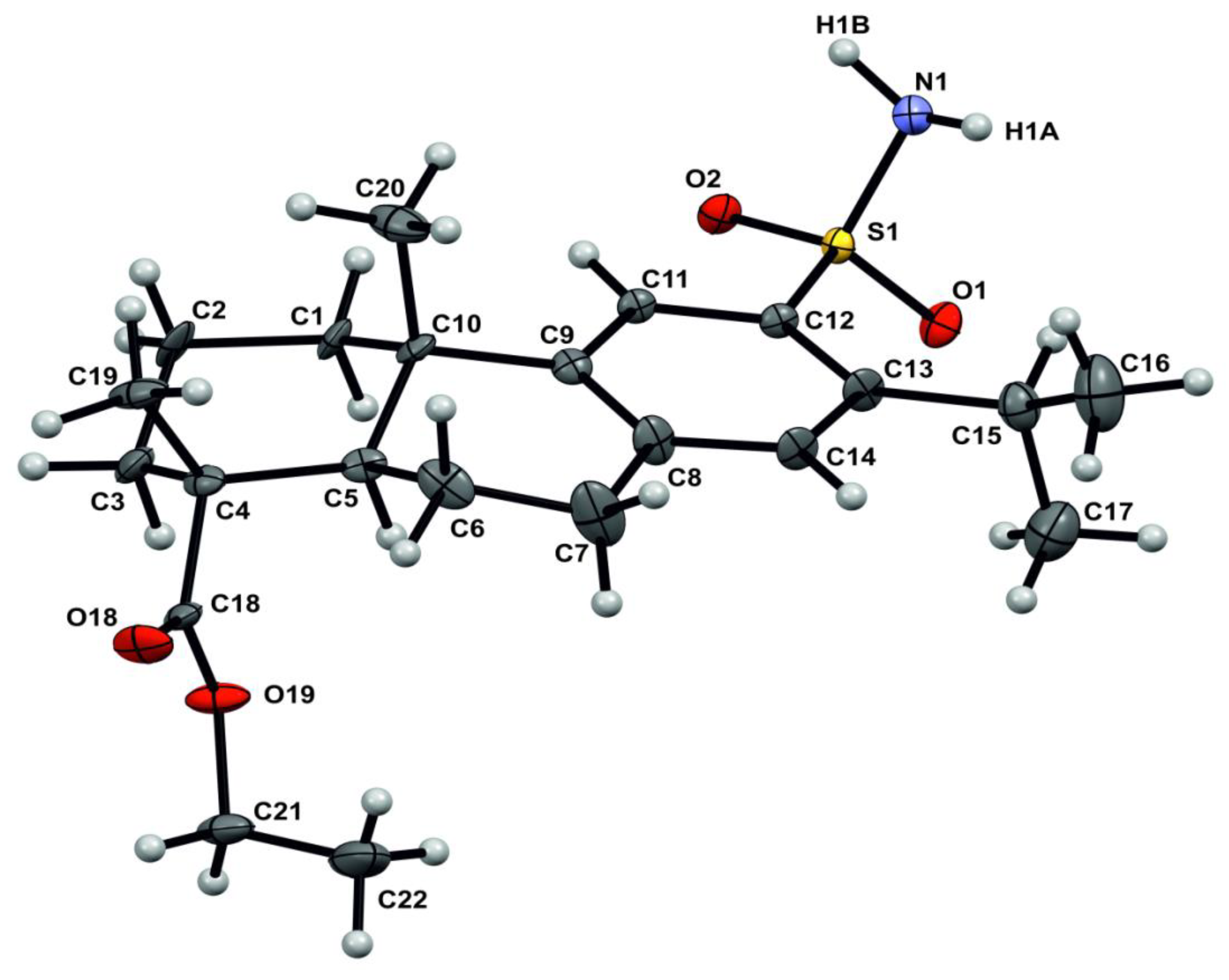
2. Results and Discussion
3. Materials and Methods
3.1. Instrumental Methods
3.2. Synthetic Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzalez, M.A. Aromatic abietane diterpenoids: Their biological activity and synthesis. Nat. Prod. Rep. 2015, 32, 684–704. [Google Scholar] [CrossRef] [PubMed]
- Izmest’ev, Y.S.; Pestova, S.V.; Lezina, O.M.; Rubtsova, S.A.; Kutchin, A.V. Synthesis of novel chiral 18-sulfanyl and sulfonyl dehydroabietane derivatives. ChemistrySelect 2019, 4, 11034–11037. [Google Scholar] [CrossRef]
- Pestova, S.V.; Petukhov, D.V.; Izmest’ev, E.S.; Rubtsova, S.A. Synthesis of dehydroabietane-derived sulfonamides with a lysine fragment. Russ. J. Org. Chem. 2022, 58, 1170–1177. [Google Scholar] [CrossRef]
- Huang, R.Z.; Liang, G.B.; Huang, X.C.; Zhang, B.; Zhou, M.M.; Liao, Z.X.; Wang, H.S. Discovery of dehydroabietic acid sulfonamide based derivatives as selective matrix metalloproteinases inactivators that inhibit cell migration and proliferation. Eur. J. Med. Chem. 2017, 138, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Kodato, S.; Kawamori, M.; Morikawa, T.; Nakai, H.; Takeda, M.; Saito, S.; Onoda, Y.; Tamaki, H. Antiulcer activity of dehydroabietic acid derivatives. Chem. Pharm. Bull. 1985, 33, 1472–1487. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Shibat, K.; Hongo, A.; Kinoshita, M. Ecabet sodium, a locally acting antiulcer drug, inhibits urease activity of Helicobacter pylori. Eur. J. Pharmacol. 1998, 345, 193–198. [Google Scholar] [CrossRef]
- APEX (Version 2.1), SAINTPlus, Data Reduction and Correction Program, Version 7.31A, Bruker Advanced X-ray Solutions; BrukerXS Inc.: Madison, WI, USA, 2006.
- Sheldrick, G.M. SADABS; University of Göttingen: Gottingen, Germany, 2004. [Google Scholar]
- Sheldrick, G.M. SHELXT: Integrating space group determination and structure solution. Acta Crystallogr. A Found. Adv. 2014, 70, 1437–1442. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Edgington, P.R.; McCabe, P.; Macrae, C.F.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Van De Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
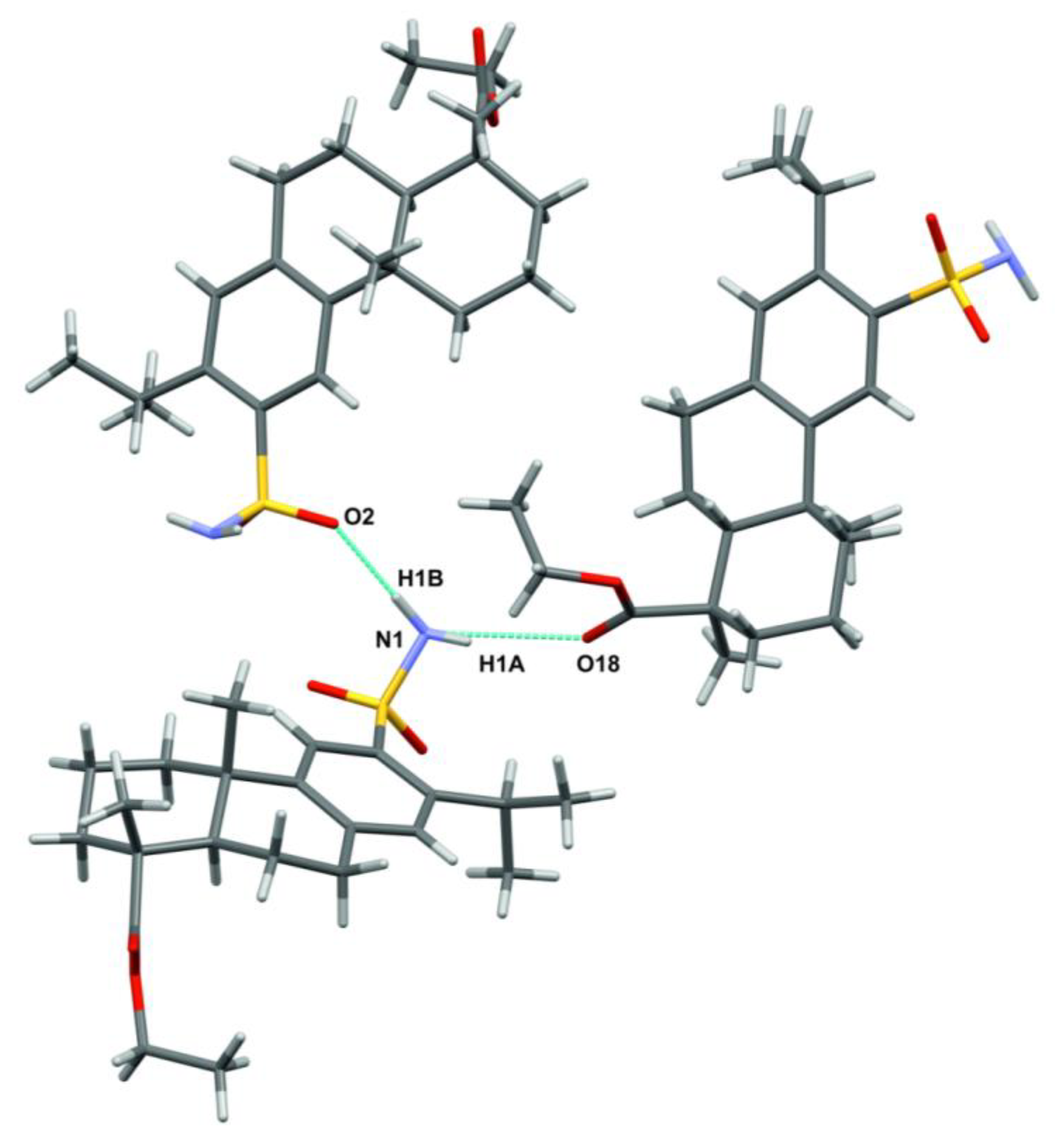
| H-Bond | N-H, Å | H···O, Å | N···O, Å | ∠N-H···O, ° | Symmetric Transformation |
|---|---|---|---|---|---|
| N1-H1A···O18 | 0.83(10) | 2.26(10) | 3.046(11) | 159(8) | 1/2 − x, 1 − y, 1/2 + z |
| N1-H1B···O2 | 0.95(11) | 2.02(11) | 2.963(11) | 179(12) | −1/2 + x, 3/2 − y, 1 − z |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izmest’ev, E.S.; Pestova, S.V.; Gerasimova, D.P.; Babaeva, O.B.; Lodochnikova, O.A.; Nikitina, L.E.; Kayumov, A.R.; Rubtsova, S.A. Ethyl 12-Sulfamoyl-abieta-8,11,13-trien-18-oate. Molbank 2023, 2023, M1584. https://doi.org/10.3390/M1584
Izmest’ev ES, Pestova SV, Gerasimova DP, Babaeva OB, Lodochnikova OA, Nikitina LE, Kayumov AR, Rubtsova SA. Ethyl 12-Sulfamoyl-abieta-8,11,13-trien-18-oate. Molbank. 2023; 2023(1):M1584. https://doi.org/10.3390/M1584
Chicago/Turabian StyleIzmest’ev, Evgeniy S., Svetlana V. Pestova, Darya P. Gerasimova, Olga B. Babaeva, Olga A. Lodochnikova, Liliya E. Nikitina, Airat R. Kayumov, and Svetlana A. Rubtsova. 2023. "Ethyl 12-Sulfamoyl-abieta-8,11,13-trien-18-oate" Molbank 2023, no. 1: M1584. https://doi.org/10.3390/M1584
APA StyleIzmest’ev, E. S., Pestova, S. V., Gerasimova, D. P., Babaeva, O. B., Lodochnikova, O. A., Nikitina, L. E., Kayumov, A. R., & Rubtsova, S. A. (2023). Ethyl 12-Sulfamoyl-abieta-8,11,13-trien-18-oate. Molbank, 2023(1), M1584. https://doi.org/10.3390/M1584







