Carboxybetaine and Carboxybetaine Ester Derivatives of Tetra(dodecyloxyphenyl)-calix[4]resorcinarene: Synthesis, Self-Assembly and In Vitro Toxicity
Abstract
1. Introduction
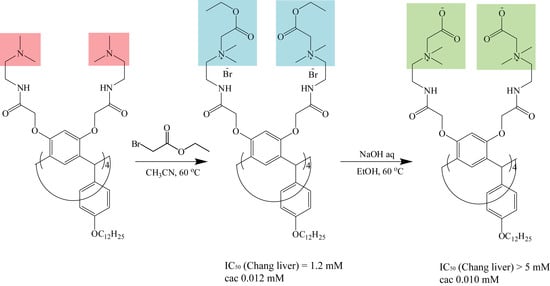
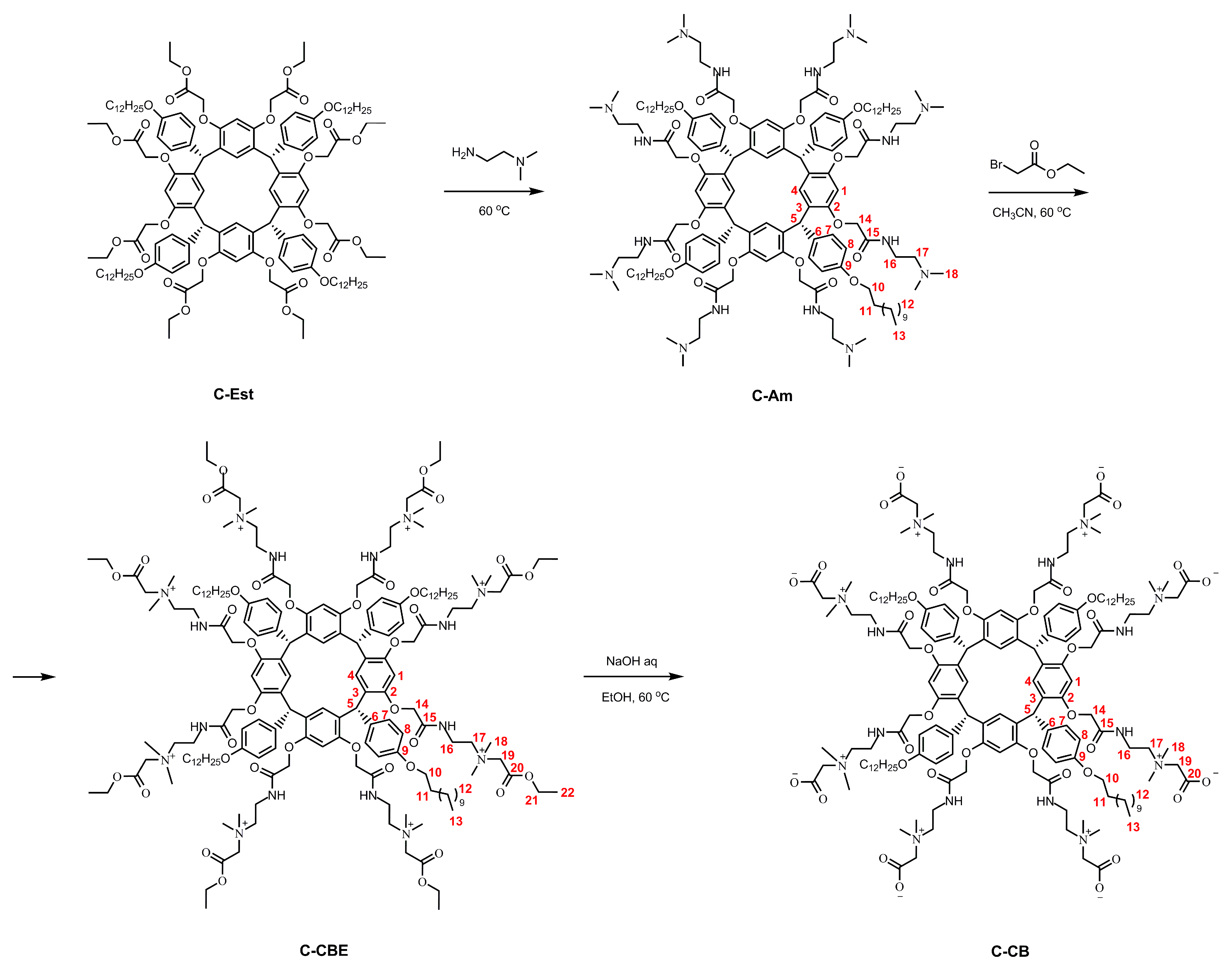
2. Results and Discussion
3. Materials and Methods
3.1. Hemolytic Activity Assay
3.2. Cytotoxic Effect Assay
3.3. The Determination of cac Values of Compounds by Fluorimetry Method with Pyrene Probe
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Español, E.S.; Villamil, M.M. Calixarenes: Generalities and their role in improving the solubility, biocompatibility, stability, bioavailability, detection, and transport of biomolecules. Biomolecules 2019, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Gu, A.; Wheate, N.J. Macrocycles as drug-enhancing excipients in pharmaceutical formulations. J. Incl. Phenom. Macrocycl. Chem. 2021, 100, 55–69. [Google Scholar] [CrossRef]
- Gao, L.; Wang, H.; Zheng, B.; Huang, F. Combating antibiotic resistance: Current strategies for the discovery of novel antibacterial materials based on macrocycle supramolecular chemistry. Giant 2021, 7, 100066. [Google Scholar] [CrossRef]
- Jain, V.K.; Kanaiya, P.H. Chemistry of calix[4]resorcinarenes. Russ. Chem. Rev. 2011, 80, 75–102. [Google Scholar] [CrossRef]
- Kashapova, N.E.; Kashapov, R.R.; Ziganshina, A.Y.; Amerhanova, S.K.; Lyubina, A.P.; Voloshina, A.D.; Salnikov, V.V.; Zakharova, L.Y. Self-assembling nanoparticles based on acetate derivatives of calix[4]resorcinol and octenidine dihydrochloride for tuning selectivity in cancer cells. Colloids Surf. A 2022, 654, 130087. [Google Scholar] [CrossRef]
- Kashapova, N.E.; Kashapov, R.R.; Ziganshina, A.Y.; Amerhanova, S.K.; Lyubina, A.P.; Voloshina, A.D.; Salnikov, V.V.; Zakharova, L.Y. Complexation-induced nanoarchitectonics of sulfonate cailx[4]resorcinol substituted at the upper rim by N-methyl-D-glucamine fragments: Morphological transition and in vitro anticancer activity. Colloids Surf. A 2022, 643, 128796. [Google Scholar] [CrossRef]
- Kashapov, R.R.; Razuvayeva, Y.S.; Ziganshina, A.Y.; Mukhitova, R.K.; Sapunova, A.S.; Voloshina, A.D.; Nizameev, I.R.; Kadirov, M.K.; Zakharova, L.Y. Design of N-methyl-D-glucamine-based resorcin[4]arene nanoparticles for enhanced apoptosis effects. Mol. Pharm. 2020, 17, 40–49. [Google Scholar] [CrossRef]
- Morozova, J.E.; Myaldzina, C.R.; Voloshina, A.D.; Lyubina, A.P.; Amerhanova, S.K.; Syakaev, V.V.; Kazakova, E.K.; Ziganshina, A.Y.; Antipin, I.S. Calixresorcine cavitands bearing lipophilic cationic fragments in the construction of mitochondrial-targeting supramolecular nanoparticles. Colloids Surf. A 2022, 642, 128622. [Google Scholar] [CrossRef]
- Shumatbaeva, A.M.; Morozova, J.E.; Syakaev, V.V.; Zakharychev, D.V.; Sapunova, A.S.; Voloshina, A.D.; Bekmuratova, F.A.; Babaev, V.M.; Antipin, I.S. A novel salt-responsive hydrogel on the base of calixresorcinarene–mPEG amide conjugate. Colloids Surf. A 2021, 611, 125814. [Google Scholar] [CrossRef]
- Shumatbaeva, A.M.; Morozova, J.E.; Syakaev, V.V.; Shalaeva, Y.V.; Sapunova, A.S.; Voloshina, A.D.; Gubaidullin, A.T.; Bazanova, O.B.; Babaev, V.M.; Nizameev, I.R.; et al. The pH-responsive calix[4]resorcinarene-mPEG conjugates bearing acylhydrazone bonds: Synthesis and study of the potential as supramolecular drug delivery systems. Colloids Surf. A 2020, 589, 124453. [Google Scholar] [CrossRef]
- Shumatbaeva, A.M.; Morozova, J.E.; Shalaeva, Y.V.; Gubaidullin, A.T.; Saifina, A.F.; Syakaev, V.V.; Bazanova, O.B.; Sapunova, A.S.; Voloshina, A.D.; Nizameev, I.R.; et al. The novel calix[4]resorcinarene-PEG conjugate: Synthesis, self-association and encapsulation properties. Colloids Surf. A 2019, 570, 182–190. [Google Scholar] [CrossRef]
- Ermakova, A.M.; Morozova, J.E.; Shalaeva, Y.V.; Syakaev, V.V.; Gubaidullin, A.T.; Voloshina, A.D.; Zobov, V.V.; Nizameev, I.R.; Bazanova, O.B.; Antipin, I.S.; et al. Nanoconjugates of a calixresorcinarene derivative with methoxy poly(ethylene glycol) fragments for drug encapsulation. Beilstein. J. Nanotechnol. 2018, 9, 2057–2070. [Google Scholar] [CrossRef] [PubMed]
- Morozova, J.E.; Gilmullina, Z.R.; Voloshina, A.D.; Lyubina, A.P.; Amerhanova, S.K.; Syakaev, V.V.; Babaeva, O.B.; Ziganshina, A.Y.; Mukhametzyanov, T.A.; Samorodov, A.V.; et al. Calix[4]resorcinarene carboxybetaines and carboxybetaine esters: Synthesis, investigation of in vitro toxicity, anti-platelet effects, anticoagulant activity, and BSA binding affinities. Int. J. Mol. Sci. 2022, 23, 15298. [Google Scholar] [CrossRef] [PubMed]
- Morozova, J.E.; Syakaev, V.V.; Kazakova, E.K.; Shalaeva, Y.V.; Nizameev, I.R.; Kadirov, M.K.; Voloshina, A.D.; Zobov, V.V.; Konovalov, A.I. Amphiphilic calixresorcinarene associates as effective solubilizing agents for hydrophobic organic acids: Construction of nano-aggregates. Soft Matter. 2016, 12, 5590–5599. [Google Scholar] [CrossRef] [PubMed]
- Syakaev, V.V.; Morozova, J.E.; Bogdanov, A.V.; Shalaeva, Y.V.; Ermakova, A.M.; Voloshina, A.D.; Zobov, V.V.; Nizameev, I.R.; Kadirov, M.K.; Mironov, V.F.; et al. Solubilization of azo-dye-modified isatin derivative by amphiphilic carboxyresorcinarenes: The effect of macrocycle structure on the supramolecular association. Colloids Surf. A 2018, 553, 368–377. [Google Scholar] [CrossRef]
- Kashapov, R.R.; Kharlamov, S.V.; Sultanova, E.D.; Mukhitova, R.K.; Kudryashova, Y.R.; Zakharova, L.Y.; Ziganshina, A.Y.; Konovalov, A.I. Controlling the size and morphology of supramolecular assemblies of viologen-resorcin[4]arene cavitands. Chem. Eur. J. 2014, 20, 14018–14025. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Gibadullina, E.M.; Burilov, A.R.; Kashapov, R.R.; Zhiltsova, E.P.; Syakaev, V.V.; Habicher, W.D.; Rümmeli, M.H.; Latypov, S.K.; Konovalov, A.I.; et al. Amphiphilic O-functionalized calix[4]resocinarenes with tunable structural behaviour. RSC Adv. 2014, 4, 9912–9919. [Google Scholar] [CrossRef]
- Bevilacqua, P.; Nuzzo, S.; Torino, E.; Condorelli, G.; Salvatore, M.; Grimaldi, A.M. Antifouling strategies of nanoparticles for diagnostic and therapeutic application: A systematic review of the literature. Nanomaterials 2021, 11, 780. [Google Scholar] [CrossRef]
- Racovita, S.; Trofin, M.A.; Loghin, D.F.; Zaharia, M.M.; Bucatariu, F.; Mihai, M.; Vasiliu, S. Polybetaines in biomedical applications. Int. J. Mol. Sci. 2021, 22, 9321. [Google Scholar] [CrossRef]
- Jiang, S.; Cao, Z. Ultralow-Fouling, functionalizable, and hydrolysable zwitterionic materials and their derivatives for biological applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef]
- Laschewsky, A. Structures and synthesis of zwitterionic polymers. Polymers 2014, 6, 1544–1601. [Google Scholar] [CrossRef]
- Ilčíková, M.; Tkáč, J.; Kasák, P. Switchable materials containing polyzwitterion moieties. Polymers 2015, 7, 2344–2370. [Google Scholar] [CrossRef]
- Podyachev, S.N.; Burmakina, N.E.; Syakaev, V.V.; Sudakova, S.N.; Shagidullin, R.R.; Konovalov, A.I. Synthesis, IR and NMR characterization and ion extraction properties of tetranonylcalix[4]resorcinol bearing acetylhydrazone groups. Tetrahedron 2009, 65, 408–417. [Google Scholar] [CrossRef]
- Shao, Q.; Jiang, S. Molecular understanding and design of zwitterionic materials. Adv. Mater. 2015, 27, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, J.; Carpena, P.; Molina-Bolívar, J.A.; Ruiz, C.C. On the determination of the critical micelle concentration by the pyrene 1: 3 ratio method. J. Colloid Interface Sci. 2003, 258, 116–122. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Cationic antimicrobial polymers and their assemblies. Int J Mol Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef]
- Wu, J. The enhanced permeability and retention (EPR) effect: The significance of the concept and methods to enhance its application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]

| HC50 (µM) | IC50 (µM) | |||
|---|---|---|---|---|
| WI-38 | Chang Liver | M-HeLa | ||
| C-CBE | 500 ± 40 | 1800 ± 100 | 1200 ± 90 | 3900 ± 300 |
| C-CB | >5000 | 2900 ± 200 | >5000 | >5000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morozova, J.E.; Gilmullina, Z.R.; Syakaev, V.V.; Voloshina, A.D.; Lyubina, A.P.; Amerhanova, S.K.; Babaeva, O.B.; Babaev, V.M.; Antipin, I.S. Carboxybetaine and Carboxybetaine Ester Derivatives of Tetra(dodecyloxyphenyl)-calix[4]resorcinarene: Synthesis, Self-Assembly and In Vitro Toxicity. Molbank 2023, 2023, M1562. https://doi.org/10.3390/M1562
Morozova JE, Gilmullina ZR, Syakaev VV, Voloshina AD, Lyubina AP, Amerhanova SK, Babaeva OB, Babaev VM, Antipin IS. Carboxybetaine and Carboxybetaine Ester Derivatives of Tetra(dodecyloxyphenyl)-calix[4]resorcinarene: Synthesis, Self-Assembly and In Vitro Toxicity. Molbank. 2023; 2023(1):M1562. https://doi.org/10.3390/M1562
Chicago/Turabian StyleMorozova, Julia E., Zuchra R. Gilmullina, Victor V. Syakaev, Alexandra D. Voloshina, Anna P. Lyubina, Syumbelya K. Amerhanova, Olga B. Babaeva, Vasily M. Babaev, and Igor S. Antipin. 2023. "Carboxybetaine and Carboxybetaine Ester Derivatives of Tetra(dodecyloxyphenyl)-calix[4]resorcinarene: Synthesis, Self-Assembly and In Vitro Toxicity" Molbank 2023, no. 1: M1562. https://doi.org/10.3390/M1562
APA StyleMorozova, J. E., Gilmullina, Z. R., Syakaev, V. V., Voloshina, A. D., Lyubina, A. P., Amerhanova, S. K., Babaeva, O. B., Babaev, V. M., & Antipin, I. S. (2023). Carboxybetaine and Carboxybetaine Ester Derivatives of Tetra(dodecyloxyphenyl)-calix[4]resorcinarene: Synthesis, Self-Assembly and In Vitro Toxicity. Molbank, 2023(1), M1562. https://doi.org/10.3390/M1562