2-Furyl-6-nitro-1,2,4-triazolo [1,5-a]pyrimidin-7-one
Abstract
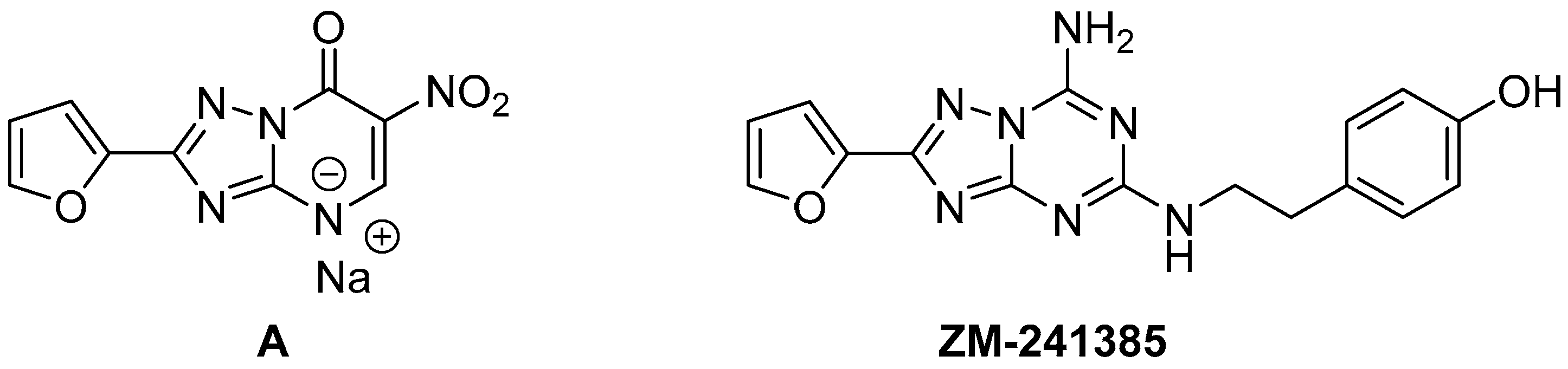
1. Introduction
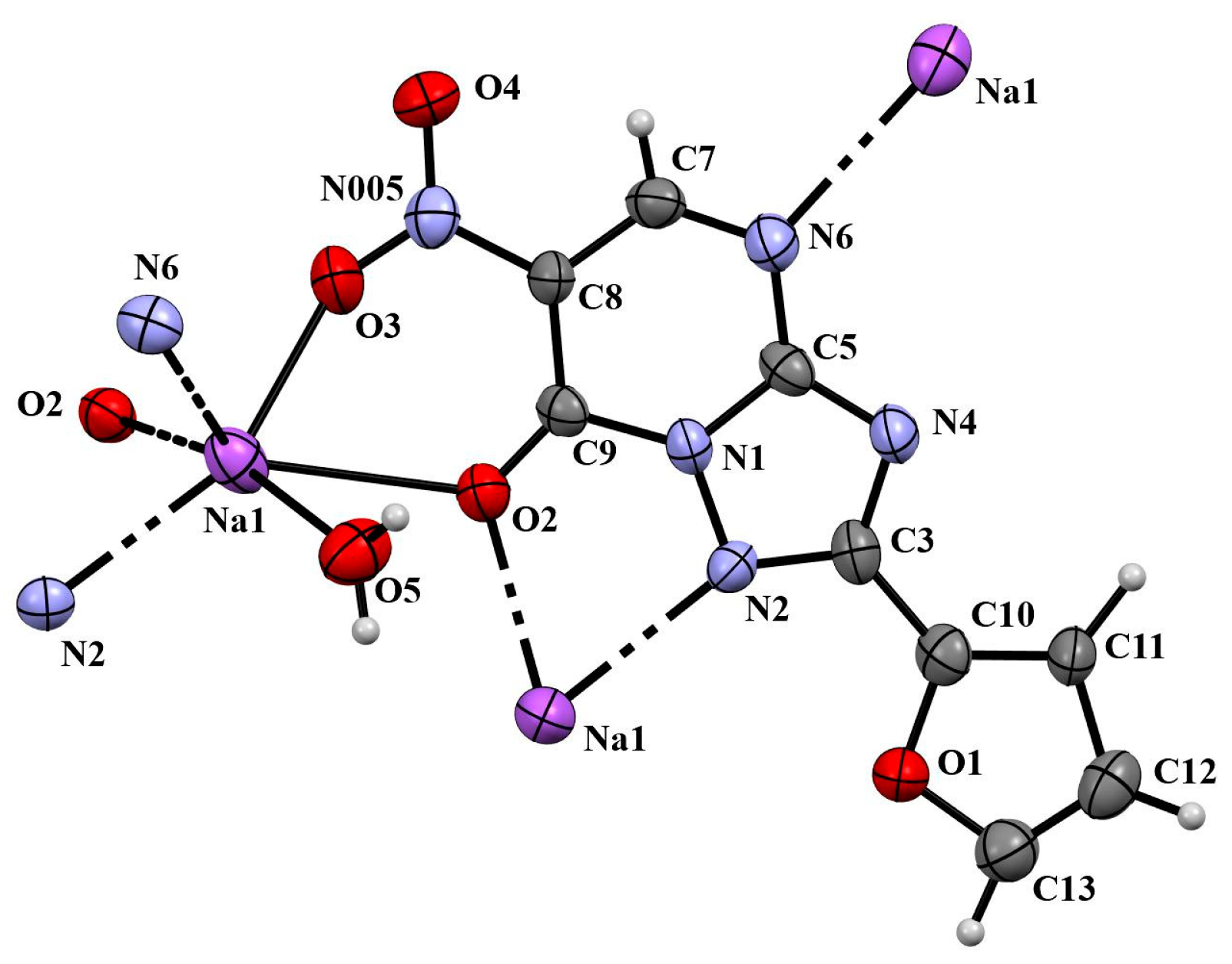
2. Results
3. Materials and Methods
3.1. Pyridinium 2-(Fur-2-yl)-6-nitro-1,2,4-triazolo[1,5-a]pyrimidin-7-One (3)
3.2. 2-(Fur-2-yl)-6-nitro-1,2,4-triazolo[1,5-a]pyrimidin-7-one Sodium Salt (A)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of Severe Sepsis in the United States: Analysis of Incidence, Outcome, and Associated Costs of Care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P. Cardiovascular Management of Septic Shock. Crit. Care Med. 2003, 31, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The Epidemiology of Sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). J. Am. Med. Assoc. 2016, 315, 762. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Volbeda, M.; Wetterslev, J.; Gluud, C.; Zijlstra, J.G.; van der Horst, I.C.C.; Keus, F. Glucocorticosteroids for Sepsis: Systematic Review with Meta-Analysis and Trial Sequential Analysis. Intensive Care Med. 2015, 41, 1220–1234. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.; Sitkovsky, M. Role of G-Protein-Coupled Adenosine Receptors in Downregulation of Inflammation and Protection from Tissue Damage. Nature 2001, 414, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Savateev, K.V.; Ulomsky, E.N.; Fedotov, V.V.; Rusinov, V.L.; Sivak, K.V.; Lyubishin, M.M.; Kuzmich, N.N.; Aleksandrov, A.G. 6-Nitrotriazolo [1,5-a]Pyrimidines as Promising Structures for Pharmacotherapy of Septic Conditions. Russ. J. Bioorg. Chem. 2017, 43, 421–428. [Google Scholar] [CrossRef]
- Hasko, G.; Nemeth, Z.; Bleich, D.; Deitch, E. Sepsis Prevention through Adenosine Receptor Modulation. WO Patent 2006 083949, 1 February 2006. [Google Scholar]
- Aghazadeh Tabrizi, M.; Baraldi, P.G.; Ruggiero, E.; Saponaro, G.; Baraldi, S.; Poli, G.; Tuccinardi, T.; Ravani, A.; Vincenzi, F.; Borea, P.A.; et al. Synthesis and Structure Activity Relationship Investigation of Triazolo [1,5-a]Pyrimidines as CB2 Cannabinoid Receptor Inverse Agonists. Eur. J. Med. Chem. 2016, 113, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedotov, V.V.; Savateev, K.V.; Ulomsky, E.N.; Drokin, R.A.; Slepukhin, P.A.; Rusinov, V.L. 2-Furyl-6-nitro-1,2,4-triazolo [1,5-a]pyrimidin-7-one. Molbank 2023, 2023, M1563. https://doi.org/10.3390/M1563
Fedotov VV, Savateev KV, Ulomsky EN, Drokin RA, Slepukhin PA, Rusinov VL. 2-Furyl-6-nitro-1,2,4-triazolo [1,5-a]pyrimidin-7-one. Molbank. 2023; 2023(1):M1563. https://doi.org/10.3390/M1563
Chicago/Turabian StyleFedotov, Victor V., Konstantin V. Savateev, Evgeny N. Ulomsky, Roman A. Drokin, Pavel A. Slepukhin, and Vladimir L. Rusinov. 2023. "2-Furyl-6-nitro-1,2,4-triazolo [1,5-a]pyrimidin-7-one" Molbank 2023, no. 1: M1563. https://doi.org/10.3390/M1563
APA StyleFedotov, V. V., Savateev, K. V., Ulomsky, E. N., Drokin, R. A., Slepukhin, P. A., & Rusinov, V. L. (2023). 2-Furyl-6-nitro-1,2,4-triazolo [1,5-a]pyrimidin-7-one. Molbank, 2023(1), M1563. https://doi.org/10.3390/M1563




