Abstract
Reaction of methyl 3-bromo-5-cyanoisothiazole-4-carboxylate with conc. H2SO4 gave methyl 3-bromo-5-carbamoylisothiazole-4-carboxylate in 93% yield. The compound was fully characterized.
1. Introduction
Isothiazoles are five-membered heterocycles that find uses as pharmaceuticals, dyes and agrochemicals. Their synthesis, chemistry and applications have been reviewed [1]. Examples of commercial isothiazoles are methylchloroisothiazolone (MCIT), an important component of the Kathon preservatives, and the fungicide isotianil (Stout®), which is active against rice blast. Isothiazole carboxamides, in particular, have biological activity, examples of which are the aforementioned isotianil 1 [2], the nucleoside 2 [3] and the antiviral 3 [4] (Figure 1).
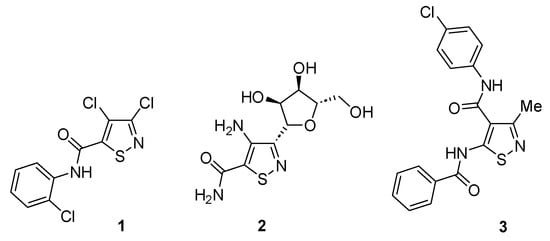
Figure 1.
Biologically active isothiazole carboxamides.
Our interest in isothiazoles focuses on their formation from 1,2,3-dithiazole precursors 4 by treatment with gaseous HCl or HBr [5] (Scheme 1). Among the products were four ester derivatives such as methyl 3-bromo-5-cyanoisothiazole-4-carboxylate (5a) (Scheme 1). This highly functionalized isothiazole offers many options for functional group modifications. Herein, we report the hydration reaction of the 5-cyano group by conc. H2SO4.
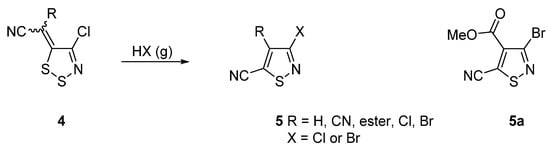
Scheme 1.
Route to isothiazole-5-carbonitriles 5 from dithiazoles 4 and the structure of methyl 3-bromo-5-cyanoisothiazole-4-carboxylate (5a).
2. Results and Discussion
The reaction of methyl 3-bromo-5-cyanoisothiazole-4-carboxylate (5a) with conc. H2SO4 at ca. 20 °C led to a slow, but complete, consumption of the starting isothiazole and isolation of the desired compound 6 in an excellent 93% yield (Scheme 2), while no other products were observed by TLC.
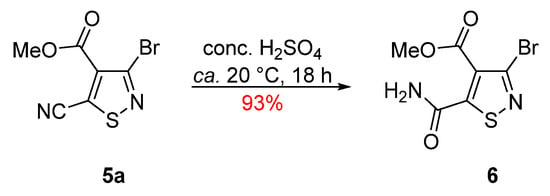
Scheme 2.
Synthesis of methyl 3-bromo-5-carbamoylisothiazole-4-carboxylate (6).
Product 6 was isolated as colorless plates, mp (DSC) onset 145.1 °C, peak max 146.5 °C (from c-hexane). UV-vis spectroscopy in dichloromethane supported an intact isothiazole ring [λmax(DCM) 286 nm, log ε 3.60], while FTIR spectroscopy showed the presence of a ν(N-H) stretch at 3370 cm−1 along with two strong ν(C=O) at 1701 and 1686 cm−1 belonging to an ester and an amide carbonyl, respectively. Mass spectrometry revealed a molecular ion (M + Na+) peak of m/z 287 (36%) along with a (M + Na+ + 2) isotope peak at 289 (33%) that supported the presence of one bromine atom. 13C NMR spectroscopy showed the presence of one CH3 resonance and five quaternary carbon resonances, among which two typical of ester and amide carbonyls (169.5 and 163.5 ppm, respectively), while the nitrile peak of starting material 5a (at 108.5 ppm [5]) is now absent (see Supplementary Materials for the NMR spectra). Moreover, a correct elemental analysis (CHN) was obtained for the molecular formula C6H5BrN2O3S. The multifunctional nature of isothiazole 6 makes it a potentially useful synthetic scaffold.
3. Materials and Methods
The reaction mixture was monitored by TLC using commercial glass-backed thin layer chromatography (TLC) plates (Merck Kieselgel 60 F254). The plates were observed under UV light at 254 and 365 nm. The melting point was determined using a PolyTherm-A, Wagner & Munz, Kofler–Hotstage Microscope apparatus (Wagner & Munz, Munich, Germany) and a TA Instruments DSC Q1000 with samples hermetically sealed in aluminum pans under an argon atmosphere, using heating rates of 5 °C/min (DSC mp listed by onset and peak values) (TA instruments, New Castle, DE, USA). The solvent used for recrystallization is indicated after the melting point. The UV-vis spectrum was obtained using a PerkinElmer Lambda-25 UV-vis spectrophotometer (PerkinElmer, Waltham, MA, USA) and inflections are identified by the abbreviation “inf”. The IR spectrum was recorded on a Shimadzu FTIR-NIR Prestige-21 spectrometer (Shimadzu, Kyoto, Japan) with Pike Miracle Ge ATR accessory (Pike Miracle, Madison, WI, USA) and strong, medium and weak peaks are represented by s, m and w, respectively. 1H and 13C NMR spectra were recorded on a Bruker Avance 500 machine [at 500 and 125 MHz, respectively, (Bruker, Billerica, MA, USA)]. Deuterated solvents were used for homonuclear lock and the signals are referenced to the deuterated solvent peaks. Attached proton test (APT) NMR studies were used for the assignment of the 13C peaks as CH3, CH2, CH and Cq (quaternary). MALDI-TOF mass spectra were recorded on a Bruker Autoflex III Smartbeam instrument. Methyl 3-bromo-5-cyanoisothiazole-4-carboxylate (5a) [5] was prepared according to the literature procedure.
3-Bromo-5-Carbamoylisothiazole-4-Carboxylate (6)
A suspension of methyl 3-bromo-5-cyanoisothiazole-4-carboxylate (5a) (98.8 mg, 0.40 mmol) in conc. H2SO4 (4 mL) was stirred at ca. 20 °C until consumption of the starting material (TLC, 18 h). The mixture was then poured onto ice and extracted with DCM (3 × 10 mL), dried over Na2SO4 and evaporated to give the title compound 6 (98.9 mg, 93%) as colorless plates, mp (hotstage) 143–144 °C, mp (DSC) onset 145.1 °C, peak max 146.5 °C (from c-hexane); Rf 0.70 (DCM/t-BuOMe 75:25); (found: C, 27.37; H, 1.88; N, 10.63. C6H5BrN2O3S requires C, 27.19; H, 1.90; N, 10.57%); λmax(DCM)/nm 246 inf (3.77), 286 (3.60); vmax/cm−1 3370 m, 3277 w, 3225 w and 3198 w (N-H), 1701 s (C=O), 1686 s (C=O), 1647 m, 1607 s, 1522 m, 1456 w, 1437 m, 1400 m, 1310 m, 1263 s, 1134 w, 1096 m, 1001 m, 922 m, 851 m, 789 s, 781 m, 758 m; δH(500 MHz; CDCl3) 8.75 (1H, NH2), 6.22 (1H, NH2), 4.03 (3H, s, CH3); δC(125 MHz; CDCl3) 169.5 (Cq), 163.5 (Cq), 159.3 (Cq), 138.1 (Cq), 126.4 (Cq), 53.5 (CH3); m/z (MALDI-TOF) 305 (M + K+ + 2, 25%), 303 (M + K+, 25), 289 (M + Na+ +2, 33), 287 (M + Na+, 36), 193 (80), 177 (100), 137 (68).
Supplementary Materials
The following supporting information can be downloaded online: molfile, 1H and 13C NMR and IR spectra.
Author Contributions
A.S.K. and P.A.K. conceived the experiments; A.S.K. designed the experiments; A.S.K. wrote the paper; A.S.K. and P.A.K. edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Cyprus Research Promotion Foundation, grant numbers ΣΤΡAΤHΙΙ/0308/06, NEKYP/0308/02 ΥΓΕΙA/0506/19 and ΕΝΙΣΧ/0308/83.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the following organizations and companies in Cyprus for generous donations of chemicals and glassware: The State General Laboratory, the Agricultural Research Institute, the Ministry of Agriculture, MedoChemie Ltd., Medisell Ltd. and Biotronics Ltd. Furthermore, we thank the A. G. Leventis Foundation for helping to establish the NMR facility at the University of Cyprus.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Sample Availability
Samples of the compounds are available from the authors.
References
- Clerici, F.; Gelmi, M.L.; Pellegrino, S. Comprehensive Heterocyclic Chemistry III; Chapter 4.05; Joule, J., Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 4, pp. 545–633. [Google Scholar]
- Wachendorff-Neumann, U.; Dahmen, P.; Dunkel, R.; Elbe, H.-L.; Suty-Heinze, A.; Rieck, H. Fungicidal active substance combinations. W.O. Patent 110,173, 4 October 2007. [Google Scholar]
- Buffel, D.K.; Meerpoel, L.; Toppet, S.M.; Hoornaert, G.J. Synthesis of Novel Isothiazole and Isothiazolo[4,5-d]pyrimidine Analogues of the Natural C-Nucleosides Pyrazofurin and the Formycins. Nucleosides Nucleotides 1994, 13, 719–736. [Google Scholar] [CrossRef]
- Regiec, A.; Machon, Z.; Miedzybrodzki, R.; Szymaniec, S. New isothiazole derivatives: Synthesis, reactivity, physicochemical properties and pharmacological activity. Arch. Pharm. 2006, 339, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Christoforou, I.C.; Ioannidou, H.A.; Manos, M.; Koutentis, P.A. Ring transformation of (4-chloro-5H-1,2,3-dithiazol-5-ylidene)acetonitriles to 3-haloisothiazole-5-carbonitriles. RSC Adv. 2013, 4, 7735–7748. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).