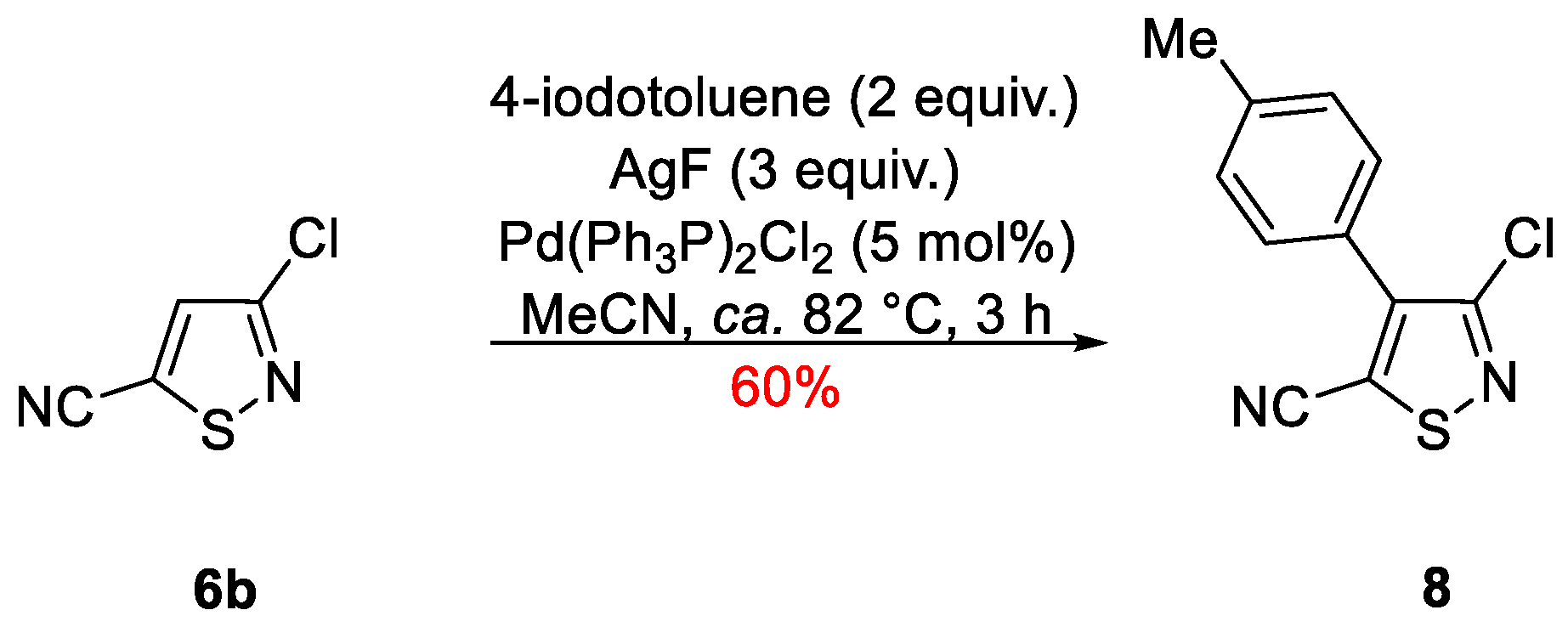
3-Chloro-4-(p-tolyl)isothiazole-5-carbonitrile
Abstract
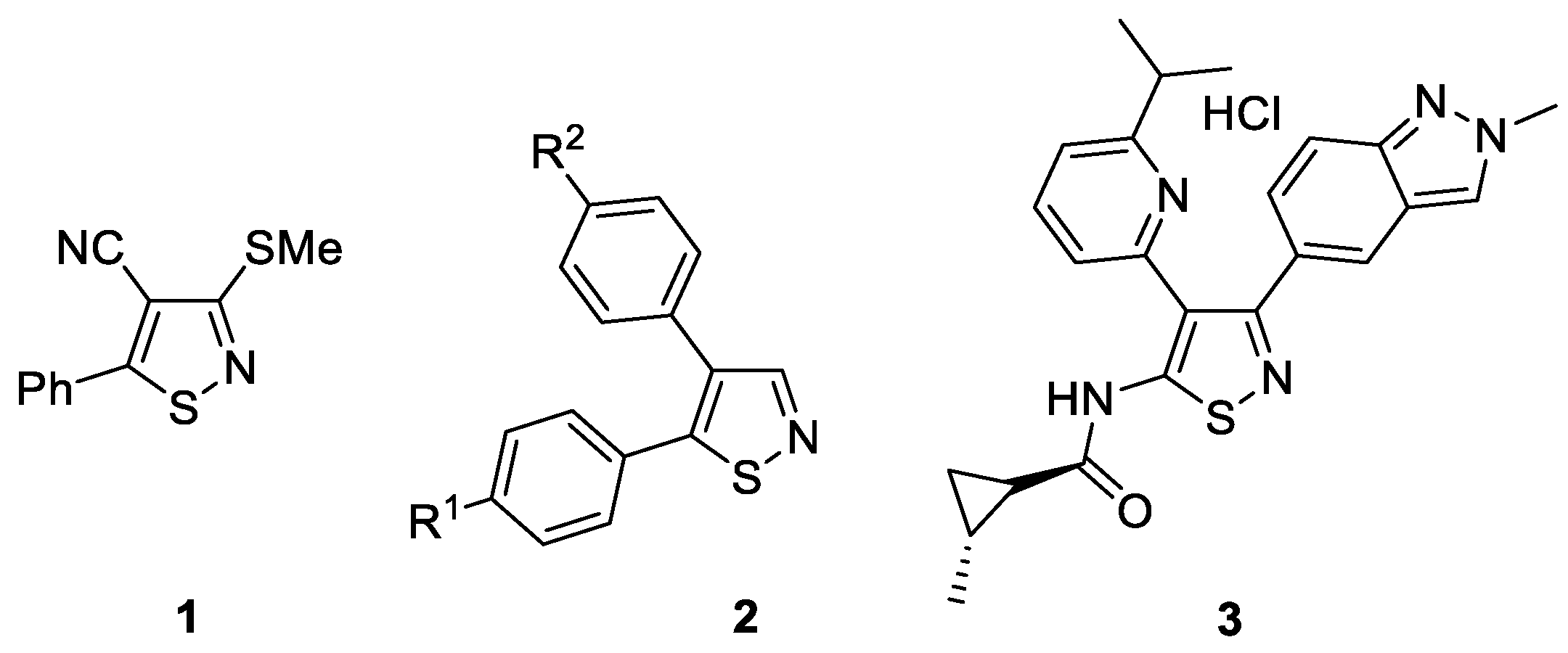
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3-Chloro-4-(p-tolyl)isothiazole-5-carbonitrile (8)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clerici, F.; Gelmi, M.L.; Pellegrino, S. Comprehensive Heterocyclic Chemistry III; Joule, J., Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 4, pp. 545–633, Chapter 4.05. [Google Scholar]
- Garozzo, A.; Stivala, A.; Tempera, G.; Castro, A. Antipoliovirus activity and mechanism of action of 3-methylthio-5-phenyl-4-isothiazolecarbonitrile. Antiviral Res. 2010, 88, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Ulbrich, H.K.; Soehnlein, O.; Lindbom, L.; Mattern, A.; Dannhardt, G. Diaryl-dithiolanes and -isothiazoles: COX-1/COX-2 and 5-LOX-inhibitory, OH scavenging and anti-adhesive activities. Bioorg. Med. Chem. 2009, 17, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Bracey-Walker, M.R.; Britton, T.C.; Catlow, J.T.; Dressman, B.A.; Fivush, A.M.; Hao, J.; Heinz, B.A.; Henry, S.S.; Hollinshead, S.P.; Iyengar, S.; et al. Discovery of (1R,2R)-N-(4-(6-isopropylpyridin-2-yl)-3-(2-methyl-2H-indazol-5-yl)isothiazol-5-yl)-2-methylcyclopropanecarboxamide, a potent and orally efficacious mGlu5 receptor negative allosteric modulator. Bioorg. Med. Chem. Lett. 2013, 23, 1249–1252. [Google Scholar] [CrossRef]
- Ioannidou, H.A.; Koutentis, P.A. Silver-mediated palladium-catalyzed direct C-H arylation of 3-bromoisothiazole-4-carbonitrile. Org. Lett. 2011, 13, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Koutentis, P.A. Silver mediated direct CH arylation of 3-bromoisothiazole-5-carbonitrile. Tetrahedron 2014, 70, 6796–6802. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Christoforou, I.C.; Ioannidou, H.A.; Manos, M.; Koutentis, P.A. Ring transformation of (4-chloro-5H-1,2,3-dithiazol-5-ylidene)acetonitriles to 3-haloisothiazole-5-carbonitriles. RSC Adv. 2013, 4, 7735–7748. [Google Scholar] [CrossRef]
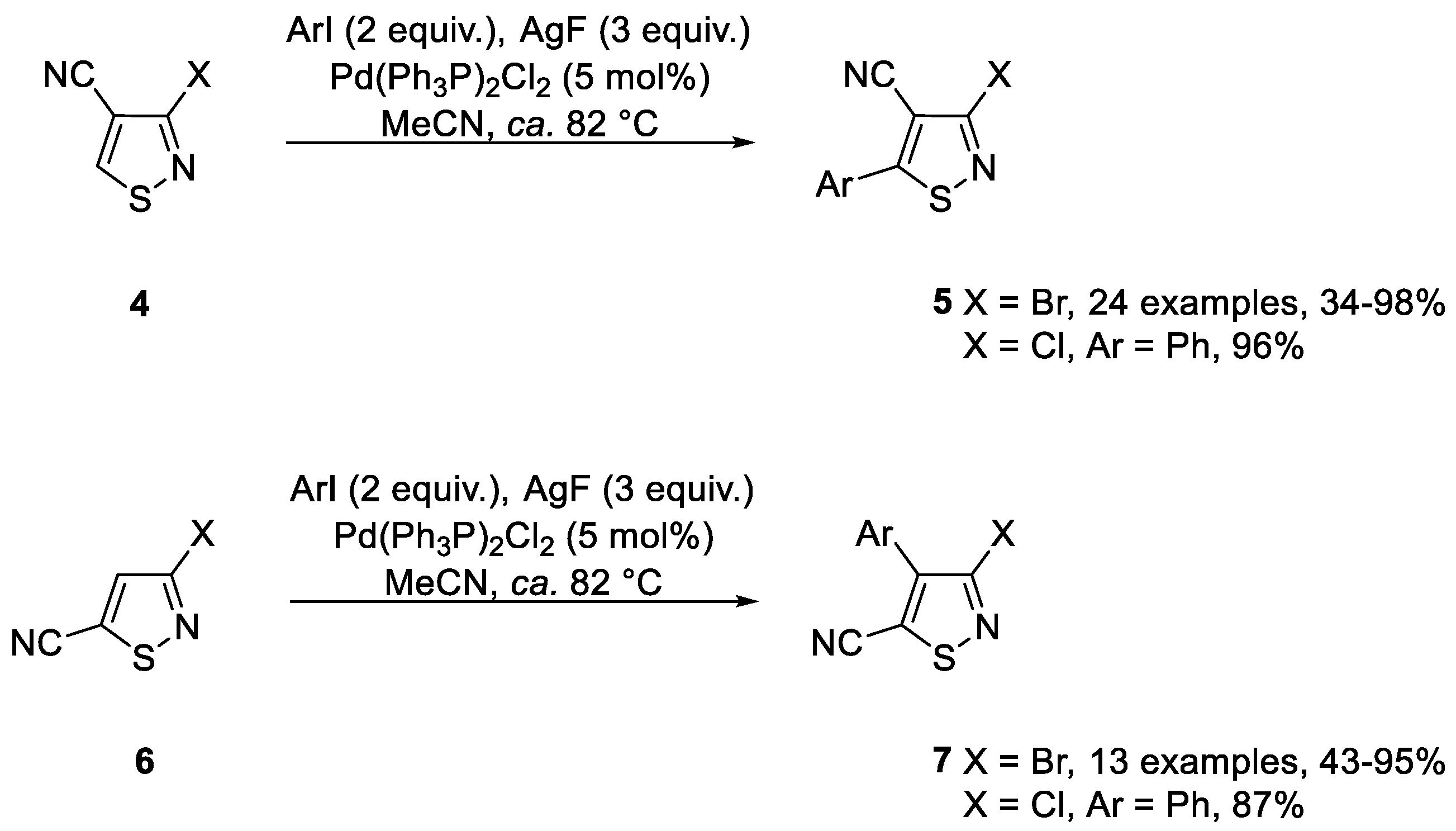
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalogirou, A.S.; Koutentis, P.A. 3-Chloro-4-(p-tolyl)isothiazole-5-carbonitrile. Molbank 2023, 2023, M1553. https://doi.org/10.3390/M1553
Kalogirou AS, Koutentis PA. 3-Chloro-4-(p-tolyl)isothiazole-5-carbonitrile. Molbank. 2023; 2023(1):M1553. https://doi.org/10.3390/M1553
Chicago/Turabian StyleKalogirou, Andreas S., and Panayiotis A. Koutentis. 2023. "3-Chloro-4-(p-tolyl)isothiazole-5-carbonitrile" Molbank 2023, no. 1: M1553. https://doi.org/10.3390/M1553
APA StyleKalogirou, A. S., & Koutentis, P. A. (2023). 3-Chloro-4-(p-tolyl)isothiazole-5-carbonitrile. Molbank, 2023(1), M1553. https://doi.org/10.3390/M1553





