1-(2-Benzyl-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octan-2-yl)ethan-1-ol
Abstract
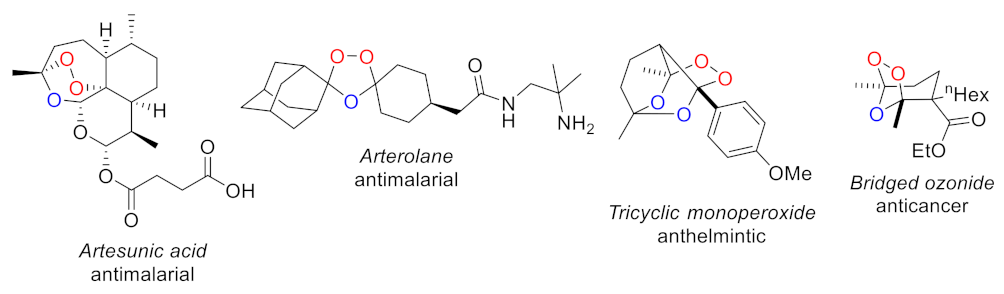
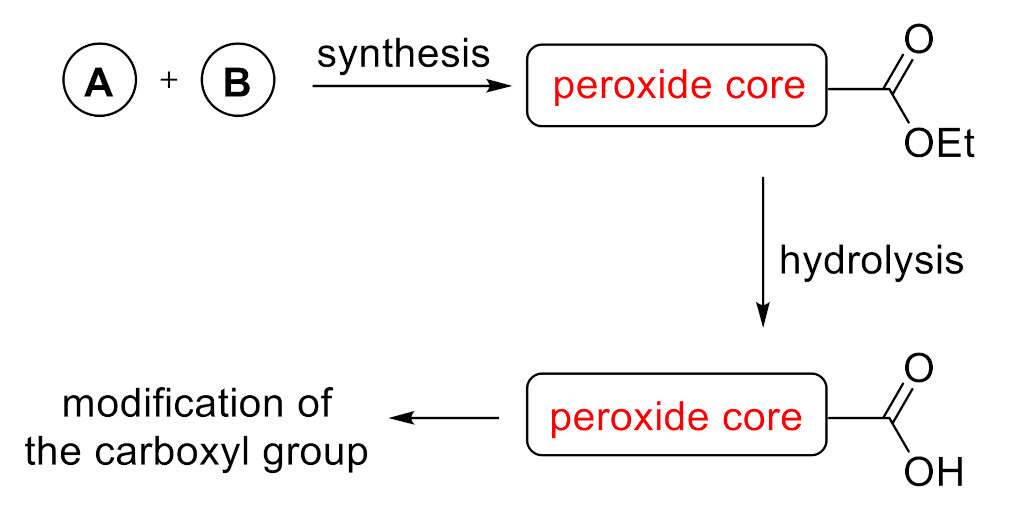
1. Introduction
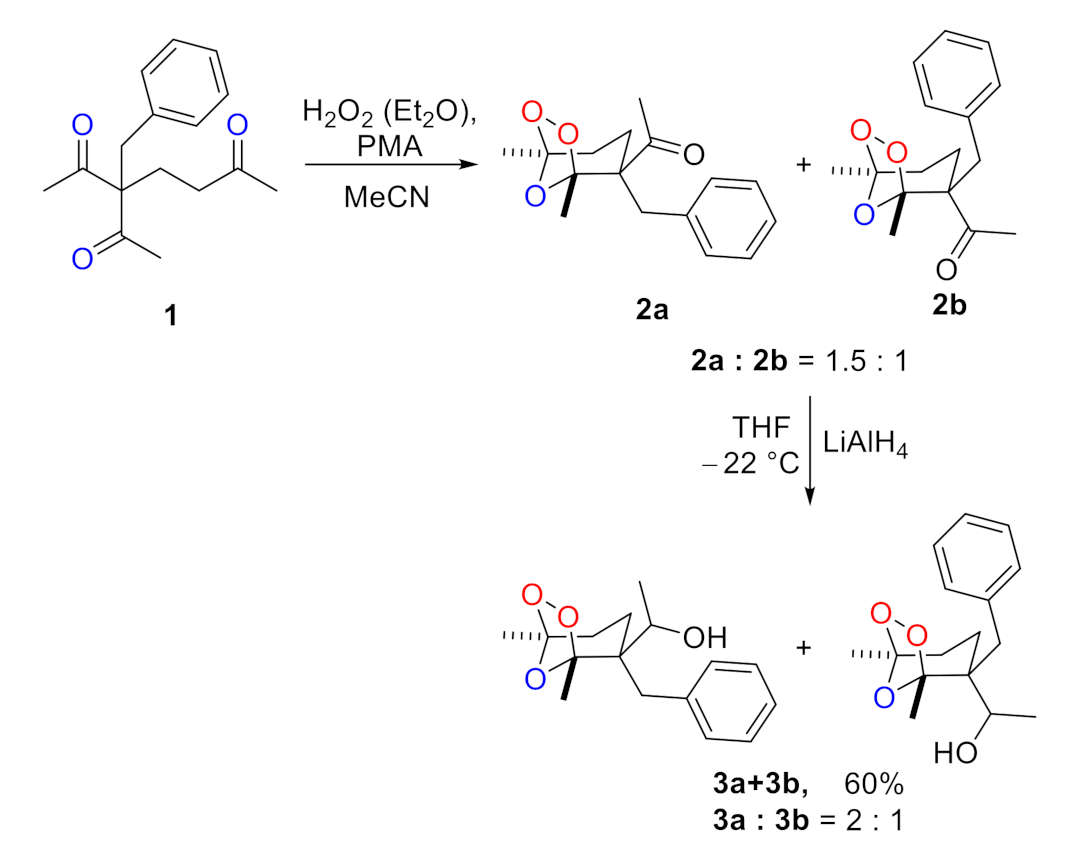
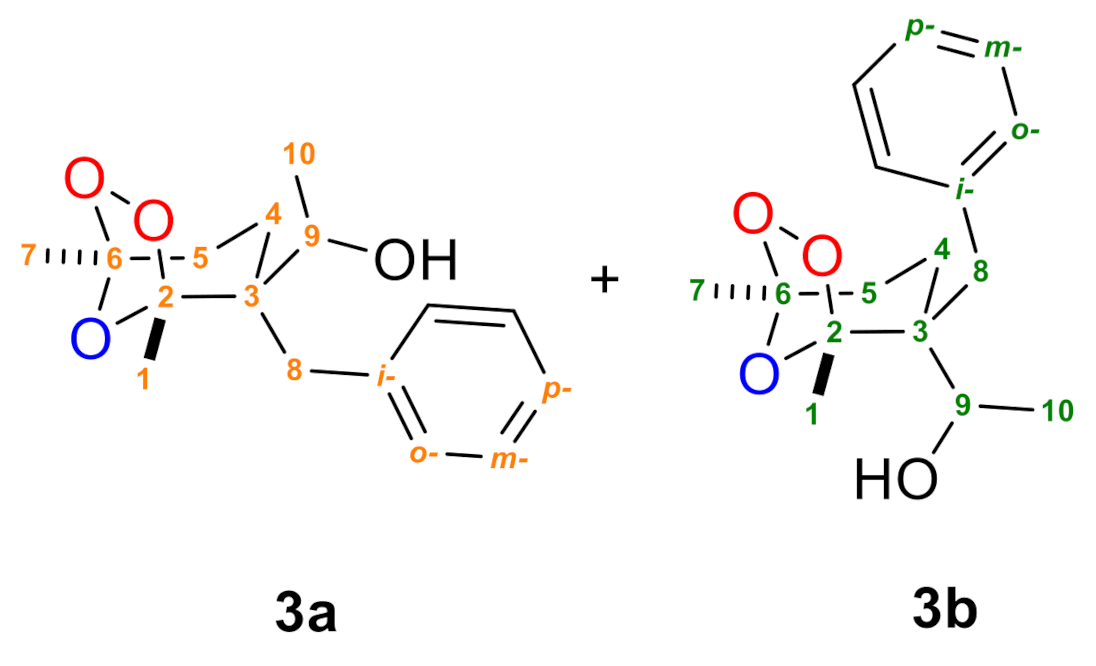
2. Results and Discussions
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slack, R.D.; Jacobine, A.M.; Posner, G.H. Antimalarial peroxides: Advances in drug discovery and design. MedChemComm 2012, 3, 281–297. [Google Scholar] [CrossRef]
- Pinet, A.; Cojean, S.; Nguyen, L.T.; Vasquez-Ocmin, P.; Maciuk, A.; Loiseau, P.M.; Le Pape, P.; Figadere, B.; Ferrie, L. Anti-protozoal and anti-fungal evaluation of 3,5-disubstituted 1,2-dioxolanes. Bioorg. Med. Chem. Lett. 2021, 47, 128196. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, G.; Giannangelo, C.; De Paoli, A.; Schuh, A.K.; Heimsch, K.C.; Anderson, D.; Brown, T.G.; MacRaild, C.A.; Wu, J.; Wang, X.; et al. Peroxide Antimalarial Drugs Target Redox Homeostasis in Plasmodium falciparum Infected Red Blood Cells. ACS Infect. Dis. 2022, 8, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Y.; Guan, W.; Su, W.; Li, G.; Zhang, S.; Yao, H. Spiral molecules with antimalarial activities: A review. Eur. J. Med. Chem. 2022, 237, 114361. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, C.; Sharma, B.; Mishra, A.; Agarwal, D.; Kannan, D.; Held, J.; Singh, S.; Awasthi, S.K. N-sulfonylpiperidinedispiro-1,2,4,5-tetraoxanes exhibit potent in vitro antiplasmodial activity and in vivo efficacy in mice infected with P. berghei ANKA. Eur. J. Med. Chem. 2022, 244, 114774. [Google Scholar] [CrossRef]
- Li, S.; Xu, W.; Wang, H.; Tang, T.; Ma, J.; Cui, Z.; Shi, H.; Qin, T.; Zhou, H.; Li, L.; et al. Ferroptosis plays an essential role in the antimalarial mechanism of low-dose dihydroartemisinin. Biomed. Pharmacother. 2022, 148, 112742. [Google Scholar] [CrossRef]
- Tiwari, M.K.; Chaudhary, S. Artemisinin Analogues as a Novel Class of Antimalarial Agents: Recent Developments, Current Scenario and Future Perspectives. In Frontiers in Drug Design Discovery; Bentham Science Publishers: Singapore, 2022; Volume 11, pp. 75–115. [Google Scholar]
- Abrams, R.P.; Carroll, W.L.; Woerpel, K.A. Five-Membered Ring Peroxide Selectively Initiates Ferroptosis in Cancer Cells. ACS Chem. Biol. 2016, 11, 1305–1312. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Syroeshkin, M.A.; Levitsky, D.O.; Fleury, F.; Terent’ev, A.O. Cyclic peroxides as promising anticancer agents: In vitro cytotoxicity study of synthetic ozonides and tetraoxanes on human prostate cancer cell lines. Med. Chem. Res. 2017, 26, 170–179. [Google Scholar] [CrossRef]
- Chaudhari, M.B.; Moorthy, S.; Patil, S.; Bisht, G.S.; Mohamed, H.; Basu, S.; Gnanaprakasam, B. Iron-Catalyzed Batch/Continuous Flow C-H Functionalization Module for the Synthesis of Anticancer Peroxides. J. Org. Chem. 2018, 83, 1358–1368. [Google Scholar] [CrossRef]
- Vil’, V.A.; Yaremenko, I.A.; Fomenkov, D.I.; Levitsky, D.O.; Fleury, F.; Terent’ev, A.O. Ion exchange resin-catalyzed synthesis of bridged tetraoxanes possessing in vitro cytotoxicity against HeLa cancer cells. Chem. Heterocycl. Comp. 2020, 56, 722–726. [Google Scholar] [CrossRef]
- Makhmudiyarova, N.N.; Ishmukhametova, I.R.; Dzhemileva, L.U.; Tyumkina, T.V.; D’yakonov, V.A.; Ibragimov, A.G.; Dzhemilev, U.M. Synthesis and anticancer activity novel dimeric azatriperoxides. RSC Adv. 2019, 9, 18923–18929. [Google Scholar] [CrossRef] [PubMed]
- Coghi, P.; Yaremenko, I.A.; Prommana, P.; Wu, J.N.; Zhang, R.L.; Ng, J.P.L.; Belyakova, Y.Y.; Law, B.Y.K.; Radulov, P.S.; Uthaipibull, C.; et al. Antimalarial and Anticancer Activity Evaluation of Bridged Ozonides, Aminoperoxides, and Tetraoxanes. ChemMedChem 2022, 17, e202200328. [Google Scholar] [CrossRef] [PubMed]
- Yaremenko, I.A.; Syromyatnikov, M.Y.; Radulov, P.S.; Belyakova, Y.Y.; Fomenkov, D.I.; Popov, V.N.; Terent’ev, A.O. Cyclic Synthetic Peroxides Inhibit Growth of Entomopathogenic Fungus Ascosphaera apis without Toxic Effect on Bumblebees. Molecules 2020, 25, 1954. [Google Scholar] [CrossRef] [PubMed]
- Yaremenko, I.A.; Radulov, P.S.; Belyakova, Y.Y.; Demina, A.A.; Fomenkov, D.I.; Barsukov, D.V.; Subbotina, I.R.; Fleury, F.; Terent’ev, A.O. Catalyst Development for the Synthesis of Ozonides and Tetraoxanes Under Heterogeneous Conditions: Disclosure of an Unprecedented Class of Fungicides for Agricultural Application. Chem. Eur. J. 2020, 26, 4734–4751. [Google Scholar] [CrossRef]
- Keiser, J.; Utzinger, J.; Tanner, M.; Dong, Y.; Vennerstrom, J.L. The synthetic peroxide OZ78 is effective against Echinostoma caproni and Fasciola hepatica. J. Antimicrob. Chemother. 2006, 58, 1193–1197. [Google Scholar] [CrossRef]
- Zhao, Q.; Vargas, M.; Dong, Y.; Zhou, L.; Wang, X.; Sriraghavan, K.; Keiser, J.; Vennerstrom, J.L. Structure-activity relationship of an ozonide carboxylic acid (OZ78) against Fasciola hepatica. J. Med. Chem. 2010, 53, 4223–4233. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Q.; Vargas, M.; Dong, Y.; Sriraghavan, K.; Keiser, J.; Vennerstrom, J.L. The activity of dispiro peroxides against Fasciola hepatica. Bioorg. Med. Chem. Lett. 2011, 21, 5320–5323. [Google Scholar] [CrossRef]
- Cowan, N.; Yaremenko, I.A.; Krylov, I.B.; Terent’ev, A.O.; Keiser, J. Elucidation of the in vitro and in vivo activities of bridged 1,2,4-trioxolanes, bridged 1,2,4,5-tetraoxanes, tricyclic monoperoxides, silyl peroxides, and hydroxylamine derivatives against Schistosoma mansoni. Bioorg. Med. Chem. 2015, 23, 5175–5181. [Google Scholar] [CrossRef]
- Amado, P.S.M.; Costa, I.C.C.; Paixao, J.A.; Mendes, R.F.; Cortes, S.; Cristiano, M.L.S. Synthesis, Structure and Antileishmanial Evaluation of Endoperoxide-Pyrazole Hybrids. Molecules 2022, 27, 5401. [Google Scholar] [CrossRef]
- Chou, S.; Marousek, G.; Auerochs, S.; Stamminger, T.; Milbradt, J.; Marschall, M. The unique antiviral activity of artesunate is broadly effective against human cytomegaloviruses including therapy-resistant mutants. Antiviral Res. 2011, 92, 364–368. [Google Scholar] [CrossRef]
- Yang, J.J.; Boissier, J.; Chen, J.L.; Yao, H.; Yang, S.; Rognon, A.; Qiao, C. Design, synthesis and biological evaluation of praziquantel and endoperoxide conjugates as antischistosomal agents. Future Med. Chem. 2015, 7, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Ge, X.; Wang, J.; Wei, Z.; Feng, W.H.; Wang, J. Ergosterol peroxide exhibits antiviral and immunomodulatory abilities against porcine deltacoronavirus (PDCoV) via suppression of NF-kappaB and p38/MAPK signaling pathways in vitro. Int. Immunopharmacol. 2021, 93, 107317. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, L.; Yaremenko, I.A.; Capci, A.; Struwe, J.; Tailor, D.; Dheeraj, A.; Hodek, J.; Belyakova, Y.Y.; Radulov, P.S.; Weber, J.; et al. Synthesis and in vitro Study of Artemisinin/Synthetic Peroxide-Based Hybrid Compounds against SARS-CoV-2 and Cancer. ChemMedChem 2022, 17, e202200005. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Hasan, M.H.; Mitra, D.; Bollavarapu, R.; Valente, E.J.; Tandon, R.; Raucher, D.; Hamme, A.T., 2nd. Design, Synthesis, and Preliminary Studies of Spiro-isoxazoline-peroxides against Human Cytomegalovirus and Glioblastoma parallel. J. Org. Chem. 2019, 84, 6992–7006. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, S.D.; Spangler, B.; Gut, J.; Lauterwasser, E.M.; Rosenthal, P.J.; Renslo, A.R. Drug delivery to the malaria parasite using an arterolane-like scaffold. ChemMedChem 2015, 10, 47–51. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Miller, H.; Knox, K.; Kundu, M.; Henrickson, K.J.; Arav-Boger, R. Inhibition of Human Coronaviruses by Antimalarial Peroxides. ACS Infect. Dis. 2021, 7, 1985–1995. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Borisov, D.A.; Yaremenko, I.A. General methods for the preparation of 1,2,4,5-tetraoxanes—Key structures for the development of peroxidic antimalarial agents. Chem. Heterocycl. Comp. 2012, 48, 55–58. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Yaremenko, I.A.; Glinushkin, A.P.; Nikishin, G.I. Synthesis of peroxides from β,δ-triketones under heterogeneous conditions. Russ. J. Org. Chem. 2015, 51, 1681–1687. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Yaremenko, I.A. Stable and Unstable 1,2-Dioxolanes: Origin, Synthesis, and Biological Activities. In Science of Synthesis Knowledge Updates; Georg Thieme Verlag KG: New York, NY, USA, 2020; Volume 2, pp. 277–314. [Google Scholar]
- Radulov, P.S.; Yaremenko, I.A. Application of BF3·Et2O in the synthesis of cyclic organic peroxides (microreview). Chem. Heterocycl. Comp. 2020, 56, 1146–1148. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Radulov, P.S.; Belyakova, Y.Y.; Fomenkov, D.I.; Tsogoeva, S.B.; Terent’ev, A.O. Lewis Acids and Heteropoly Acids in the Synthesis of Organic Peroxides. Pharmaceuticals 2022, 15, 472. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Brautigam, M.; Kleczka, M.; Raabe, A. Synthetic Approaches to Mono- and Bicyclic Perortho-Esters with a Central 1,2,4-Trioxane Ring as the Privileged Lead Structure in Antimalarial and Antitumor-Active Peroxides and Clarification of the Peroxide Relevance. Molecules 2017, 22, 119. [Google Scholar] [CrossRef] [PubMed]
- Ubale, A.S.; Chaudhari, M.B.; Shaikh, M.A.; Gnanaprakasam, B. Manganese-Catalyzed Synthesis of Quaternary Peroxides: Application in Catalytic Deperoxidation and Rearrangement Reactions. J. Org. Chem. 2020, 85, 10488–10503. [Google Scholar] [CrossRef] [PubMed]
- Makhmudiyarova, N.; Ishmukhametova, I.; Dzhemileva, L.; D’yakonov, V.; Ibragimov, A.; Dzhemilev, U. First Example of Catalytic Synthesis of Cyclic S-Containing Di- and Triperoxides. Molecules 2020, 25, 1874. [Google Scholar] [CrossRef] [PubMed]
- Eske, A.; Ecker, S.; Fendinger, C.; Goldfuss, B.; Jonen, M.; Lefarth, J.; Neudorfl, J.M.; Spilles, M.; Griesbeck, A.G. Spirofused and Annulated 1,2,4-Trioxepane-, 1,2,4-Trioxocane-, and 1,2,4-Trioxonane-Cyclohexadienones: Cyclic Peroxides with Unusual Ring Conformation Dynamics. Angew. Chem. Int. Ed. 2018, 57, 13770–13774. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Gomes, G.d.P.; Radulov, P.S.; Belyakova, Y.Y.; Vilikotskiy, A.E.; Vil’, V.A.; Korlyukov, A.A.; Nikishin, G.I.; Alabugin, I.V.; Terent’ev, A.O. Ozone-Free Synthesis of Ozonides: Assembling Bicyclic Structures from 1,5-Diketones and Hydrogen Peroxide. J. Org. Chem. 2018, 83, 4402–4426. [Google Scholar] [CrossRef]
- Shaveta; Mishra, S.; Singh, P. Hybrid molecules: The privileged scaffolds for various pharmaceuticals. Eur. J. Med. Chem. 2016, 124, 500–536. [Google Scholar] [CrossRef]
- Alkhzem, A.H.; Woodman, T.J.; Blagbrough, I.S. Design and synthesis of hybrid compounds as novel drugs and medicines. RSC Adv. 2022, 12, 19470–19484. [Google Scholar] [CrossRef]
- Decker, M. Design of Hybrid Molecules for Drug Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–338. [Google Scholar]
- Russel, A.T. Synthesis by Addition to Alkynes and Alkenes. In Category 5, Compounds with One Saturated Carbon Heteroatom Bond; Georg Thieme Verlag KG: Stuttgart, Germany, 2008. [Google Scholar]
- Story, P.R.; Bishop, C.E.; Burgess, J.R.; Murray, R.W.; Youssefyeh, R.D. Evidence for a new mechanism of ozonolysis. J. Am. Chem. Soc. 1968, 90, 1907–1909. [Google Scholar] [CrossRef]
- Bishop, C.E.; Story, P.R. Mechanisms of ozonolysis. Reductive cleavage of ozonides. J. Am. Chem. Soc. 1968, 90, 1905–1907. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Vil’, V.A.; Demchuk, D.V.; Terent’ev, A.O. Rearrangements of organic peroxides and related processes. Beilstein J. Org. Chem. 2016, 12, 1647–1748. [Google Scholar] [CrossRef]
- Dong, Y.; Wittlin, S.; Sriraghavan, K.; Chollet, J.; Charman, S.A.; Charman, W.N.; Scheurer, C.; Urwyler, H.; Santo Tomas, J.; Snyder, C.; et al. The Structure−Activity Relationship of the Antimalarial Ozonide Arterolane (OZ277). J. Med. Chem. 2009, 53, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Yaremenko, I.A.; Terent’ev, A.O.; Vil’, V.A.; Novikov, R.A.; Chernyshev, V.V.; Tafeenko, V.A.; Levitsky, D.O.; Fleury, F.; Nikishin, G.I. Approach for the preparation of various classes of peroxides based on the reaction of triketones with H2O2: First examples of ozonide rearrangements. Chem. Eur. J. 2014, 20, 10160–10169. [Google Scholar] [CrossRef] [PubMed]
- Saito, I.; Nagata, R.; Yuba, K.; Matsuura, T. Synthesis of α-silyloxyhydroperoxides from the reaction of silyl enol ethers and hydrogen peroxide. Tetrahedron Lett. 1983, 24, 1737–1740. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radulov, P.S.; Yaremenko, I.A.; Terent’ev, A.O. 1-(2-Benzyl-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octan-2-yl)ethan-1-ol. Molbank 2023, 2023, M1532. https://doi.org/10.3390/M1532
Radulov PS, Yaremenko IA, Terent’ev AO. 1-(2-Benzyl-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octan-2-yl)ethan-1-ol. Molbank. 2023; 2023(1):M1532. https://doi.org/10.3390/M1532
Chicago/Turabian StyleRadulov, Peter S., Ivan A. Yaremenko, and Alexander O. Terent’ev. 2023. "1-(2-Benzyl-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octan-2-yl)ethan-1-ol" Molbank 2023, no. 1: M1532. https://doi.org/10.3390/M1532
APA StyleRadulov, P. S., Yaremenko, I. A., & Terent’ev, A. O. (2023). 1-(2-Benzyl-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octan-2-yl)ethan-1-ol. Molbank, 2023(1), M1532. https://doi.org/10.3390/M1532







