Abstract
The title compound was synthesized and characterized by IR and NMR spectroscopy and single crystal X-ray diffraction. The analysis of the crystal packing of the title compound and its analog, bearing a nitro group instead of a nitrile one, allowed a direct comparison of two common explosophoric groups: CN and NO2. By using ΔOED-based densification approach, it is shown that the CN group is lighter in mass, less dense, and participates in intermolecular bonding to a lesser extent in comparison to the NO2 group. As a result, the cyano compound has a lower density and a looser crystal packing than the nitro analog.
1. Introduction
Nitrofurazan [1,2,3,4,5,6,7,8] and nitropyrazole [9,10,11,12,13,14,15] building blocks occupy a privileged position in energetic compounds chemistry and appear in multiple blockbuster explosives and propellant ingredients. The high densities, high enthalpies of formation, high temperature resistance, and attractive oxygen balance of these nitro compounds make furazan and pyrazole important structural units in the energetic materials industry. Combining nitrofurazan and nitropyrazole units and other explosophoric groups and fragments [16] in a single molecule is a promising approach to the creation of new high-energy compounds possessing a set of required properties. Thus, furazan–pyrazole combinations have been the subject of extensive research in recent years [17,18,19,20,21,22,23].
All properties, including chemical, physical, and energetic are encoded in the molecular and structural formulas of molecules [24]. Changing the framework, the type, number, and position of substituents is a standard tool for tuning the final properties of the molecule. Both the bonds between the atoms of a molecule and the nonbonding interactions between isolated molecules determine important properties of compounds. Elucidation of the structure–property relationship is an important research method, the results of which underlie targeted synthesis. The installation of a key framework of substituents with different molecular shapes and volumes, as well as with variations in the arrangement, provides an ideal set for evaluating the effects of intra- and intermolecular interactions on the tuning of properties.
Recently, we synthesized and characterized 3-(3,4-dinitro-1H-pyrazol-5-yl)-4-nitrofurazan (1), which crystallizes as a monohydrate [21,25] (Figure 1). By using an ΔOED-based densification approach [26,27,28], the density of individual compound 1 was determined to be as high as 1.89 g/cm3 at room temperature (1.97 g/cm3 at 100 K). In our continuing efforts of investigating compounds based on the furazan–pyrazole combinations, we synthesized and characterized an analog of compound 1, compound 5, bearing a cyano group instead of a nitro group at position 4 of the pyrazole unit. It allowed us to carry out the detailed analysis of the crystal packing of both compounds in order to compare the influence of two common explosophoric groups [16] (NO2 and CN) on the crystal structure and density.
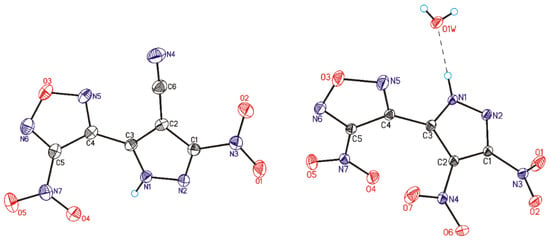
Figure 1.
General view of compounds 5 (studied in this work, shown on the left) and 1 (studied recently [25], shown on the right) showing atomic numbering. Atoms are represented as thermals ellipsoids at 50% probability. Only first independent molecules (A) are presented.
2. Results and Discussion
The synthesis route is shown in Scheme 1. Following a recently reported procedure [22], 3-amino-4-(1-amino-2,2-dicyanovinyl) furazan (3) and 3-amino-4-(5-amino-4-cyano-1H-pyrazol-3-yl)-furazan (4) were prepared starting from 3-amino-4-cyanofurazan (2). The formation of nitrofurazans [29,30] and nitropyrazoles [31,32] by oxidation of the corresponding amines is well-known, however, previously various conditions were used for these heterocycles. A mixture of 37% H2O2 with 98% H2SO4 in the presence of Na2WO4 was utilized as an oxidizing reagent. Both amino groups of compound 3 were oxidized at once, dinitro product 5 was isolated in 64% yield.
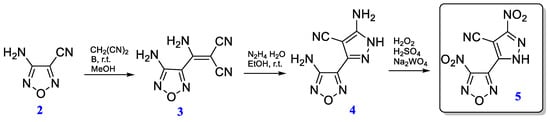
Scheme 1.
Synthetic pathway to compound 5.
Product 5 was characterized by IR, 1H, 13C, and 14N NMR spectroscopy, as well as by MS and elemental analysis (see the Supporting Information). Good quality single crystals of 3-(4-Cyano-3-nitro-1H-pyrazol-5-yl)-4-nitrofurazan (5) were grown by slow evaporation of HNO3 solution at room temperature under dry conditions. General view of 5 is depicted in Figure 1 along with its nitro analog 1. Compound 5 crystallizes in Pbca space group and asymmetric unit cell contains two molecules (A and A′). It should be noted that asymmetric unit cell of nitro analog 1 also contains two molecules along with two water molecules.
Both molecules 1 and 5 do not adopt planar structure, due, evidently, to steric hindrance caused by substituents. Selected torsion angles defining molecular conformations are provided in Table 1.

Table 1.
Selected torsion angles defining conformation of both symmetrically independent molecules (A and A′) in the crystals of 5 and 1.
The molecules of cyano compound 5 are more planar and all the substituents are nearly coplanar to corresponding heterocycles. In part, it is due to the cyano group, which is smaller than the nitro group and makes the molecules less strained. However, conformations of compounds 1 and 5 differ, which is more important. In the molecules of nitro compound 1, the heterocyclic units of the backbone adopt a transoid configuration, so that two nitro groups (at the C2 and C5 atoms) are approximately directed to the same side. This significantly distinguishes it from compound 5, the molecular backbone of which has a cisoid configuration. As a result, the hydrogen atom in 5 participates in the intramolecular H-bond N1-H1…O4 (N1′-H1′…O4′) (N-H, 0.89(2) Å, H…O 2.38(2) Å, N…O, 2.858(2) Å, <NHO, 114(2)° for molecule A; N-H, 0.87(2) Å, H…O 2.38(2) Å, N…O, 2.842(2) Å, <NHO, 113(2)° for molecule A′). On the contrary, in the nitro compound 1, acidic NH hydrogen atom seeks to find a suitable acceptor in the outside that leads to inclusion of water molecules in the unit cell. It should be noted that all our attempts to obtain unsolvated single crystal of 1 failed. In order to explain the observed conformational differences, we carried out geometry optimization of both molecules in both conformations at M052X/aug-cc-pvtz level of theory, which has been proven to provide realistic estimation of both geometry and energetic characteristics [33,34,35]. All four optimizations converged to true minima, and the calculated electron densities were analyzed in terms of the “Atom in Molecules” theory [36] using the AIMAll program [37]. The calculated structures are presented in Figure 2.
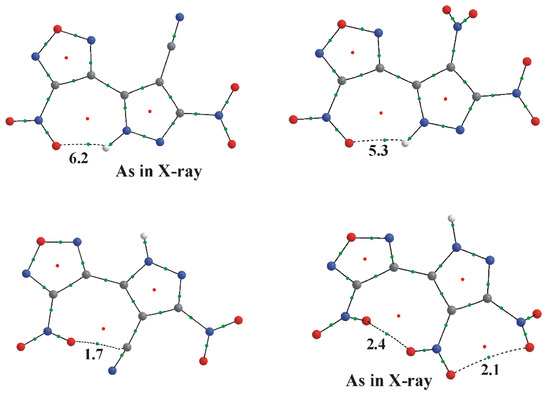
Figure 2.
Calculated conformations of molecules 5 (left) and 1 (right) showing critical points (as obtained from topological analysis; BCPs and ring CPs are shown as green and red circles, respectively) and energies for noncovalent contacts in kcal/mol. Cisoid conformations are shown at the top; transoid at the bottom. Noncovalent intramolecular interactions are shown by dashed lines.
Calculation leads to somewhat more planar furazan–pyrazole backbone, but to greater deviation of the substituents from the planes of the corresponding heterocycles (Table 2). At the same time, the energetically preferred conformations for both molecules correspond to those observed experimentally. For cyano compound 5, the cisoid form is 1.5 kcal/mol more stable than the transoid form. The reverse pattern is observed for nitro compound 1, where the transoid form appears to be more stable by 1.4 kcal/mol. Both cisoid and transoid conformations of both molecules are stabilized by additional intramolecular noncovalent contacts: H-bond for the transoid form, and π…π interactions between substituents in the cisoid one. Energies of those interactions were estimated from their correlation with the potential energy density at the bond critical point [38,39] and are presented in Figure 2. It is seen that in the cisoid conformation of 5, the N1-H1…O4 hydrogen bond is stronger (by 6.2 − 1.7 = 4.5 kcal/mol) than the interaction between the nitro and cyano groups. We cannot expect that the same difference would necessarily be in total energies due to variability of the conformation (from cisoid to transoid). At the same time, such a difference for compound 1 is only (5.3 − 2.4 − 2.1) 0.8 kcal/mol. It means that the gain in energy due to the hydrogen bond in the cisoid form is more preferable for the cyano compound 5.

Table 2.
Selected torsion angles defining conformation of calculated molecules 5 and 1 in both cisoid and transoid conformations.
Therefore, the above-described calculations demonstrate that molecular conformations observed in crystals are, in part, determined by the preferences of isolated molecules, while some deviations of the torsion angles are due to the influence of the crystal packing. In the case of nitro compound 1, it leads to the formation of hydrate, while cyano compound 5 crystallizes in an individual form. In the crystal structure of 5, hydrogen atoms (in addition to intramolecular H-bond) form relatively weak intermolecular hydrogen bonds, thereby resulting in a formation of dimeric associates (Figure 3), which are linked to each other by numerous shortened O(N)…O(N) contacts that are frequently observed in polynitrocompounds [40,41,42].
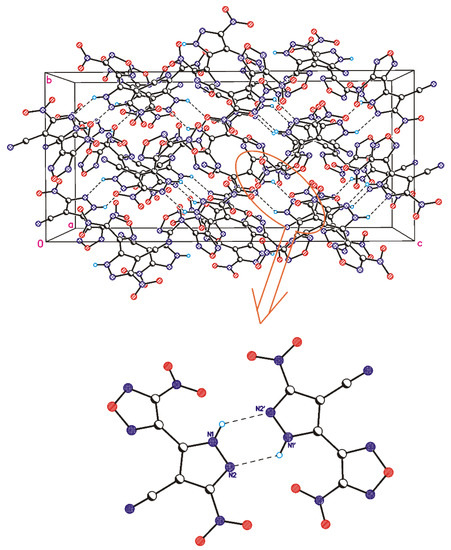
Figure 3.
Crystal packing fragment of compound 5. H-bonded dimer formed between two symmetrically independent molecules is shown at the bottom.
It is interesting to note that the density of the nitro compound (1.971 g/cm3 at 100 K) is significantly higher than that of the cyano compound (1.790 g/cm3). Based on the ΔOED densification approach, we compared contributions of molecular units to the crystal packing density, which allowed us to explain the observed difference. For each molecular unit, as well as for the whole molecules, their densities were estimated based on volumes obtained from topological analysis of calculated electron density for isolated molecules (dmol) and molecules in the crystal (dcryst). Their difference (ΔOED criterion) shows a degree of densification or, in the other words, a degree of participation of a molecule in intermolecular bonding that is closely related to the tightness of crystal packing (more detailed description of the approach is provided in Supplementary Materials file):
ΔOED = dcryst − dmol

Table 3.
Volumes (Å3), densities (g/cm3), and ΔOED (g/cm3) values of molecular units and whole molecule 5 1.

Table 4.
Volumes (Å3), densities (g/cm3), and ΔOED (g/cm3) values of molecular units and whole molecule 1 1.
First of all, it is seen that the density of isolated molecule 5 itself is lower than that of 1, which is evidently the result of a replacement of the nitro group with the cyano group that is lighter in mass and less dense. Moreover, the ΔOED criterion for the CN group is much lower than that for other molecular units. It means that the cyano group is not actively involved in intermolecular bonding, which is also reflected in lower activity and densification of the two remaining nitro groups, as well as heterocycles.
As expected, compound 5 exhibits a high detonation performance, and the calculated detonation velocity (D) and detonation pressure (P) are 7920 m⋅s−1 and 28.4 GPa, respectively, which exceeds the most widely used energetic material, TNT (D = 7450 m⋅s−1, P = 23.5 GPa).
In conclusion, cyano compound 5 was synthesized and characterized. Unlike nitro analog 1, which forms crystallohydrate, crystals of cyano compound 5 do not include a solvent. Using an ΔOED-based approach, we show that the CN group is lighter in mass and significantly less dense in comparison to the NO2 group. Moreover, the nitrile group participates in intermolecular bonding to a lesser extent. As a result, the replacement of the nitro group with a nitrile one leads to a significant decrease in density, which is clearly demonstrated by comparing cyano compound 5 with its nitro analog 1.
3. Materials and Methods
General: All the reagents were of analytical grade, purchased from commercial sources, and used as received. Starting 3-amino-4-(5-amino-4-cyano-1H-pyrazol-3-yl)-furazan (4) [22] was synthesized by using previously reported methods. Infrared spectra were recorded on a BrukerALPHA instrument (Bruker, Bremen, Germany) in KBr pellets (unless otherwise stated). Mass-spectra were recorded on a Varian MAT-311 A instrument. The 1H, 13C, and 14N NMR spectra were recorded on a Bruker AM-300 instrument at 300.13, 75.47, and 21.68 MHz, respectively, at 298 K. The chemical shifts values (δ) of 1H and 13C nuclei were reported relative to TMS, and for 14N nuclei relative to MeNO2, high-filed chemical shifts are given with a minus sign. Analytical TLC was performed using commercially pre-coated silica gel plates (Kieselgel 60 F254), and visualization was effected with short-wavelength UV light. Melting points were determined on Gallenkamp melting point apparatus and they remained uncorrected. Elemental analyses were obtained by using a CHNS/O Analyzer 2400 (Perkin–Elmer instruments Series II, Perkin Elmer, Waltham, MA, USA).
CAUTION! Although we encountered no difficulties during preparation and handling of this compound, they are potentially explosive energetic materials. Manipulations must be carried out by using appropriate standard safety precautions.
3-(4-Cyano-3-nitro-1H-pyrazol-5-yl)-4-nitrofurazan (5). A suspension of compound 4 (1.2 g, 6.28 mmol) and Na2WO4 × 2H2O (7.2 g, 21.9 mmol) in a mixture of H2O2 (37%, 59 mL) and sulfuric acid (98%, 27 mL) was heated at 50 °C and stirred for 3 h. The clear yellow solution was cooled, poured into ice water (90 mL), and extracted with ethyl acetate (6 × 40 mL). The combined organic layer was stirred with magnesium sulfate and then filtered. The solvent was removed, leaving an orange solid. The crude compound was recrystallized from water to give dinitro product 5 (1.01 g, 64%): mp 218–220 °C. IR (KBr) ν 3302, 2260, 1573, 1540, 1504, 1415, 1337, 1281, 1210, 1190, 1097, 975, 839, 828, 734 cm−1; 1H NMR (acetone-d6) 12.4 (br.s). 13C NMR (acetone-d6) δ 92.3, 109.0, 133.3, 140.1, 155.5, 159.9. 14N NMR (acetone-d6) δ −38.5. MS: m/z (%) = 251 [M+, 12], 205 (M+-NO2, 10), 175 (M+-NO2-NO, 5), 130, 100, 76, 46, 30 (100). Anal. Calcd. for C6HN7O5 (251.12): C, 28.70; H, 0.40; N, 39.04. Found: C, 28.74; H, 0.37; N, 38.98%.
Single crystal X-ray diffraction experiment of 5 was carried out using SMART APEX2 CCD diffractometer (λ(Mo-Kα) = 0.71073 Å, graphite monochromator, ω-scans) at 100 K. Collected data were processed by the SAINT and SADABS programs incorporated into the APEX2 program package [43]. The structure was solved by the direct methods and refined by the full-matrix least-squares procedure against F2 in anisotropic approximation. The refinement was carried out with the SHELXTL program [44]. The CCDC number (2217287) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif. Accessed on 7 November 2022.
Crystallographic data for 5: C6O5N7H are orthorhombic, space group Pbca: a = 11.9776(7) Å, b = 11.9751(7) Å, c = 25.9920(15) Å, V = 3728.1(4) Å3, Z = 16, M = 251.14, dcryst = 1.790 g⋅cm−3. wR2 = 0.1068 calculated on F2hkl for all 4919 independent reflections with 2θ < 57.9°, (GOF = 1.039, R = 0.0394 calculated on Fhkl for 3819 reflections with I > 2σ(I)).
Supplementary Materials
The following are available online. Detailed description of ΔOED-based approach. Figure showing NMR spectra 1H, 13C, and 14N, respectively [45,46].
Author Contributions
Conceptualization, K.Y.S.; investigation, K.Y.S. and K.V.S.; administration, K.Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The supporting data are available on request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Since all the compounds in this study are potentially dangerous, the authors do not assume responsibility for providing these samples. On the other hand, a description of the preparation of all compounds can be found in the manuscript and in the Supplementary Materials.
References
- Sheremetev, A.B.; Makhova, N.N.; Friedrichsen, W. Monocyclic Furazans and Furoxans. Adv. Heterocycl. Chem. 2001, 78, 65–188. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Makhova, N.N. 1,2,5-Oxadiazole-Based High-Energy-Density Materials: Synthesis and Performance. ChemPlusChem 2020, 85, 13–42. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, J.; Bi, F.; Wang, B. Energetic materials based on poly furazan and furoxan structures. Chin. Chem. Lett. 2020, 31, 2375–2394. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, M.J.; Zhao, W.; Qu, Y.; Xing, X.W.; Meng, J.W.; Liu, Y.C. Application and Development of 3,4-Bis(3-nitrofurazan-4-yl)furoxan (DNTF). Russ. J. Gen. Chem. 2021, 91, 445–455. [Google Scholar] [CrossRef]
- Tang, J.; Yang, H.; Cui, Y.; Cheng, G. Nitrogen-rich tricyclic-based energetic materials. Mater. Chem. Front. 2021, 5, 7108–7118. [Google Scholar] [CrossRef]
- Xiao, M.; Jin, X.; Zhou, J.; Hu, B. 1,2,5-Oxadiazole-1,2,3,4-tetrazole-based high-energy materials: Molecular design and screening. Struct. Chem. 2021, 32, 1619–1628. [Google Scholar] [CrossRef]
- Gulyaev, D.A.; Klenov, M.S.; Churakov, A.M.; Strelenko, Y.A.; Pivkina, A.N.; Tartakovsky, V.A. Synthesis of energetic compounds containing (3-nitro-1H-1,2,4-triazol-1-yl)-NNO-azoxy moiety. Russ. Chem. Bull. 2021, 70, 1599–1604. [Google Scholar] [CrossRef]
- Zhu, Y.; Ding, L.; Xu, L.; Chang, H.; Wang, X.; Zhang, X. Evaluation of the Thermal Hazard of the Oxidation Reaction in the Synthesis of 3,4-Bis(4-nitrofurazan-3-yl)furoxan. Org. Proc. Res. Dev. 2022, 26, 1389–1397. [Google Scholar] [CrossRef]
- Zaitsev, A.A.; Dalinger, I.L.; Shevelev, S.A. Dinitropyrazoles. Russ. Chem. Rev. 2009, 78, 589–627. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, Z.; Lan, D.; Jia, Q.; Liu, N.; Zhang, J.; Kou, K. Recent Advances in Synthesis and Properties of Nitrated-Pyrazoles Based Energetic Compounds. Molecules 2020, 25, 3475. [Google Scholar] [CrossRef]
- Zlotin, S.G.; Dalinger, I.L.; Makhova, N.N.; Tartakovsky, V.A. Nitro compounds as the core structures of promising energetic materials and versatile reagents for organic synthesis. Russ. Chem. Rev. 2020, 89, 1–54. [Google Scholar] [CrossRef]
- Chen, D.; Xiong, H.; Yang, H.; Tang, J.; Cheng, G. Nitropyrazole based tricyclic nitrogen-rich cation salts: A new class of promising insensitive energetic materials. FirePhysChem 2021, 1, 71–75. [Google Scholar] [CrossRef]
- Wu, B.; Yang, L.; Zhai, D.; Ma, C.; Pei, C. Facile synthesis of 4-amino-3,5-dinitropyrazolated energetic derivatives via 4-bromopyrazole and their performances. FirePhysChem 2021, 1, 76–82. [Google Scholar] [CrossRef]
- Lai, Y.; Liu, Y.; Huang, W.; Zeng, Z.; Yang, H.; Tang, Y. Synthesis and characterization of pyrazole- and imidazole- derived energetic compounds featuring ortho azido/nitro groups. FirePhysChem 2022, 2, 140–146. [Google Scholar] [CrossRef]
- Bölter, M.F.; Harter, A.; Klapötke, T.M.; Stierstorfer, J. Isomers of Dinitropyrazoles: Synthesis, comparison and Tuning of their Physicochemical Properties. ChemPlusChem 2018, 83, 804. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.L.; Wang, B.Z.; Qiu, L.L.; Xu, R.Q.; Sheremetev, A.B. Recent synthetic efforts towards high energydensity materials: How to design high-performance energetic structures? FirePhysChem 2022, 2, 83–139. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Yudin, I.L.; Palysaeva, N.V.; Suponitsky, K.Y. The Synthesis of 4-(3-Nitrofurazan-4-yl)-3,5-dinitropyrazole and its Salts. J. Heterocycl. Chem. 2012, 49, 394–401. [Google Scholar] [CrossRef]
- Dalinger, I.L.; Vatsadze, I.A.; Shkineva, T.K.; Kormanov, A.V.; Kozeev, A.M.; Averkiev, B.B.; Dalinger, A.I.; Beklemishev, M.K.; Sheremetev, A.B. Synthesis and investigation of isomeric mono- and dinitro derivatives of 3-methyl-4-(pyrazol-3-yl)furazan. Chem. Heterocycl. Compd. 2015, 51, 545–552. [Google Scholar] [CrossRef]
- Dalinger, I.L.; Suponitsky, K.Y.; Pivkina, A.N.; Sheremetev, A.B. Novel Melt-Castable Energetic Pyrazole: A Pyrazolyl-Furazan Framework Bearing Five Nitro Groups. Propellants Explos. Pyrotech. 2016, 41, 789–792. [Google Scholar] [CrossRef]
- Kormanov, A.V.; Lipilin, D.L.; Shkineva, T.K.; Vatsadze, I.A.; Kozeev, A.M.; Dalinger, I.L. Synthesis and transformations of 3(5)-(3-methylfurazan-4-yl)-4-nitro-1H-pyrazole-5(3)-carboxylic acid. Chem. Heterocycl. Comp. 2017, 53, 876–882. [Google Scholar] [CrossRef]
- Dalinger, I.L.; Shkineva, T.K.; Vatsadze, I.A.; Kormanov, A.V.; Kozeev, A.M.; Suponitsky, K.Y.; Pivkina, A.N.; Sheremetev, A.B. Novel Energetic CNO oxidizer: Pernitro-Substituted Pyrazolyl-Furazan Framework. FirePhysChem 2021, 1, 83–89. [Google Scholar] [CrossRef]
- Strizhenko, K.V.; Vasil’ev, L.S.; Suponitsky, K.Y.; Sheremetev, A.B. 3-Amino-4-(1-amino-2-cyanovinyl)furazans: Synthesis and cyclization. Chem. Heterocycl. Comp. 2020, 56, 1103–1107. [Google Scholar] [CrossRef]
- Yan, T.; Yang, C.; Ma, J.; Cheng, G.; Yang, H. Intramolecular integration of multiple heterocyclic skeletons for energetic materials with enhanced energy & safety. Chem. Eng. J. 2022, 428, 131400. [Google Scholar] [CrossRef]
- Roy, K.; Kar, S.; Das, R.N. A Primer on QSAR/QSPR Modeling; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Shkineva, T.K.; Dalinger, I.L. Density estimation method for individual compounds from X-ray diffraction analysis of their solvated forms. Chem. Heterocycl. Comp. 2022, 58, 539–542. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Smol’yakov, A.F.; Ananyev, I.V.; Khakhalev, A.V.; Gidaspov, A.A.; Sheremetev, A.B. 3,4-Dinitrofurazan: Structural Nonequivalence of Ortho -Nitro Groups as a Key Feature of the Crystal Structure and Density. ChemistrySelect 2020, 5, 14543–14548. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Anisimov, A.A.; Ananyev, I.V.; Lashakov, A.A.; Osintseva, S.V.; Zalomlenkov, V.A.; Gidaspov, A.A. On the influence of weak intermolecular interactions on the molecular crystal density of 1,3,5-triazine trinitroalkyl derivatives. Chem. Heterocycl. Comp. 2021, 57, 266–273. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Fedyanin, I.V.; Karnoukhova, V.A.; Zalomlenkov, V.A.; Gidaspov, A.A.; Bakharev, V.V.; Sheremetev, A.B. Energetic Co-Crystal of a Primary Metal-Free Explosive with BTF. Ideal Pair for Co-Crystallization. Molecules 2021, 26, 7452. [Google Scholar] [CrossRef]
- Novikova, T.S.; Melnikova, T.M.; Kharitonova, O.V.; Kulagina, V.O.; Aleksandrova, N.S.; Sheremetev, A.B.; Pivina, T.S.; Khmelnitskii, L.I.; Novikov, S.S. An Effective Method for the Oxidation of Aminofurazans to Nitrofurazans. Mendeleev Commun. 1994, 4, 138–140. [Google Scholar] [CrossRef]
- Sheremetev, A.B. Efficient synthesis of nitrofurazans using HOF • MeCN. Russ. Chem. Bull. 2022, 71, 1818–1820. [Google Scholar] [CrossRef]
- Vinogradov, V.M.; Cherkasova, T.I.; Dalinger, I.L.; Shevelev, S.A. Nitropyrazoles. 5. Synthesis of 4-substituted 3-nitropyrazoles from 3-amino-4-pyrazolecarbonitrile. Russ. Chem. Bull. 1993, 42, 1552–1554. [Google Scholar] [CrossRef]
- Zhao, X.X.; Zhang, J.C.; Li, S.H.; Yang, Q.P.; Li, Y.C.; Pang, S.P. A Green and Facile Approach for Synthesis of Nitro Heteroaromatics in Water. Org. Process Res. Dev. 2014, 18, 886–890. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Aleksandrova, N.S.; Suponitsky, K.Y.; Antipin, M.Y.; Tartakovsky, V.A. One-pot synthesis of 4,6,8-trinitro-4,5,7,8-tetrahydro-6H-furazano[3,4-f]-1,3,5-triazepine in ionic liquids. Mendeleev. Commun. 2010, 20, 249–252. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Aleksandrova, N.S.; Semyakin, S.S.; Suponitsky, K.Y.; Lempert, D.B. Synthesis and Characterization of 3-(5-(Fluorodinitromethyl)-1H-1,2,4-triazol-3-yl)-4-nitrofurazan: A Novel Promising Energetic Component of Boron-based Fuels for Rocket Ramjet Engines. Chem. Asian J. 2019, 14, 4255–4261. [Google Scholar] [CrossRef] [PubMed]
- Dmitrienko, A.O.; Karnoukhova, V.A.; Potemkin, A.A.; Struchkova, M.I.; Kryazhevskikh, I.A.; Suponitsky, K.Y. The influence of halogen type on structural features of compounds containing α-halo-α,α-dinitroethyl moieties. Chem. Heterocycl. Comp. 2017, 53, 532–539. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules. A Quantum Theory; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Keith, T.A. AIMAll (Version 15.05.18); TK Gristmill Software: Overland Park, KS, USA, 2015. [Google Scholar]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Rozas, I.; Elguero, J.; Molins, E. About the evaluation of the local kinetic, potential and total energy densities in closed-shell interactions. Chem. Phys. Lett. 2001, 336, 457–461. [Google Scholar] [CrossRef]
- Göbel, M.; Klapötke, T.M. Development and Testing of Energetic Materials: The Concept of High Densities Based on the Trinitroethyl Functionality. Adv. Funct. Mater. 2009, 19, 347–365. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Yudin, I.L.; Yu, K. Suponitsky, Ionic liquid-assisted synthesis of trinitroethyl esters. Mendeleev Commun. 2006, 16, 264–266. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Aleksandrova, N.S.; Palysaeva, N.V.; Struchkova, M.I.; Tartakovsky, V.A.; Suponitsky, K.Y. Ionic Liquids as Unique Solvents in One-Pot Synthesis of 4-( N,2,2,2-Tetranitroethylamino)-3-R-Furazans. Chem. Eur. J. 2013, 19, 12446–12457. [Google Scholar] [CrossRef]
- APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Kudin, K.N., Jr.; Burant, J.C.; Millam, J.M.; et al. A. Gaussian 03, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2004.
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).