Abstract
The title compound, 1-(2-Benzyl-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octan-2-yl)ethan-1-ol, was synthesized for the first time by the selective reduction in keto ozonide under the action of the strong reducing agent LiAlH4. The product was characterized by NMR, IR, HRMS, and elemental analysis.
1. Introduction
Cyclic organic peroxides are attractive compounds for drug discovery. Their importance was illustrated by the 2015 Nobel Prize in Medicine awarded to Youyou Tu for the discovery and development of the natural antimalarial peroxide artemisinin. Recent studies have demonstrated that cyclic synthetic peroxides exhibit antimalarial [1,2,3,4,5,6,7], anticancer [8,9,10,11,12,13], antifungal [14,15], antiparasitic, [16,17,18,19,20] and antiviral [21,22,23,24,25] activities. Notably, synthetic peroxide arterolane (Figure 1), which contains the ozonide core, is used in medical practice for the treatment of malaria [26]. Moreover, arterolane shows in vitro activity against α-coronavirus NL63, and β-coronavirus SARS-CoV-2 [27].
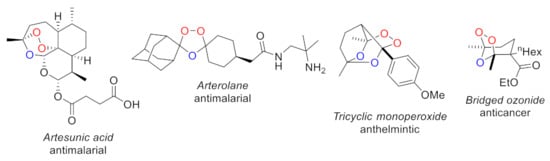
Figure 1.
Bioactive peroxides.
In light of the previous information, the expansion of the structural diversity of peroxides is an important task for the development of biologically active compounds based on them. [28,29,30,31,32,33,34,35,36,37]. One of the solutions to this problem is the synthesis of peroxides with functional groups and their transformation with the preservation of the O–O bond. This approach can provide access to hybrid molecules. The synthesis of hybrid molecules is a modern trend in medicinal chemistry [38,39,40].
Herein we report the synthesis of bridged ozonides containing a hydroxyl functional group via selective reduction in keto ozonide by a strong reducing agent LiAlH4. This is very unusual because the ozonide cycle has proven to be moderately stable under such harsh conditions. Typically, peroxides are not resistant to reducing agents and decompose to form alcohols [41,42,43].
2. Results and Discussions
Transformations of peroxides with preservation of the peroxide cycle are nontrivial processes. Under the action of acid or base, peroxides can undergo transformations with cleavage of the O-O bond, for example, the Baeyer–Villiger, Criegee, Udris–Sergeev/Hock or Kornblum DeLaMare reactions and related processes [44]. Usually, the expansion of the structural diversity of peroxides is based on the following sequence: synthesis of peroxide with an ester group → hydrolysis of the ester group with the formation of a carboxyl group → modification of the carboxyl group (Scheme 1) [45].
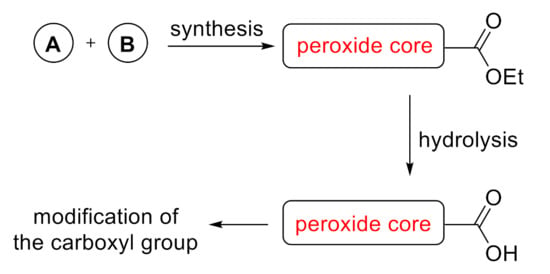
Scheme 1.
General approach to modification of peroxides.
Another promising approach to expand the diversity of peroxides is the introduction of a hydroxyl group by reduction in the ester or keto group. However, here it is necessary to keep a balance in which the peroxide cycle will be preserved, and the ester or keto group will be converted to alcohol. In this study, we found the key to ozonides 3a + 3b containing the OH-functional group (Scheme 2).
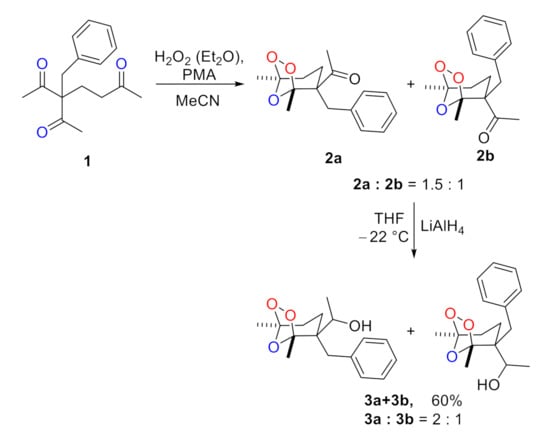
Scheme 2.
Synthesis of ozonides 3a + 3b.
The starting keto ozonides 2a + 2b were synthesized by phosphomolybdic acid-catalyzed peroxidation of β,δ’-triketone 1 [46]. The molar ratio of 2a:2b was 1.5:1. Then, the resulting mixture of keto ozonides 2a + 2b was treated with LiAlH4 in THF at −22 °C to give the title compounds 3a + 3b which were formed as the mixture of diastereomers in a 60% isolated yield with the molar ratio of 3a:3b = 2:1. The stereochemical assignment was based upon NMR analysis with 2D correlation spectroscopic techniques (HSQC, HMBC and NOESY). Surprisingly, the ozonide cycle was found to be moderately resistant to LiAlH4. This fact may open up additional possibilities in the modification of peroxides for the development of effective biologically active compounds. Structure of 3a + 3b was confirmed by NMR, IR spectroscopy, HRMS, and elemental analysis.
3. Materials and Methods
Caution! Although we encountered no difficulties in working with peroxides, precautions, such as the use of shields, fume hoods, and the avoidance of transition metal salts, heating, and shaking, should be taken whenever possible.
1H and 13C NMR spectra were taken with a Bruker AM-300 machine (Bruker AXS Handheld Inc., Kennewick, WA, USA) (at frequencies of 300 and 75 MHz) in CDCl3 solution with TMS as the standard. J values are given in Hz. The high-resolution MS spectrum was measured on a Bruker microTOF II instrument (Bruker Daltonik Gmbh, Bremen, Germany) using electrospray ionization (ESI). The elemental analysis was performed on a 2400 Elemental Analyzer (Perkin ElmerInc., Waltham, MA, USA). The TLC analysis was carried out on silica gel chromatography plates Macherey-Nagel Alugram UV254; sorbent: Silica 60, specific surface (BET) ~500 m2/g, mean pore size 60 Å, specific pore volume 0.75 mL/g, particle size 5–17 µm; binder: highly polymeric product, which is stable in almost all organic solvents and resistant towards aggressive visualiz ation reagents. Chromatography of peroxides was performed on silica gel (0.040–0.060 mm, 60 A, CAS 7631-86-9). The solvents and reagents were purchased from commercial sources. A solution of H2O2 in Et2O (6.0 M) was prepared by the extraction with Et2O (5 × 100 mL) from a 35% aqueous solution (100 mL) followed by drying over MgSO4. Then, part of Et2O was removed in the vacuum of a membrane vacuum pump at 20–25 °C and titrated iodometrically [15,47].
Synthesis of 1-(2-benzyl-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octan-2-yl)ethan-1-ol (3a + 3b) (Figure 2) (Supplementary Materials).
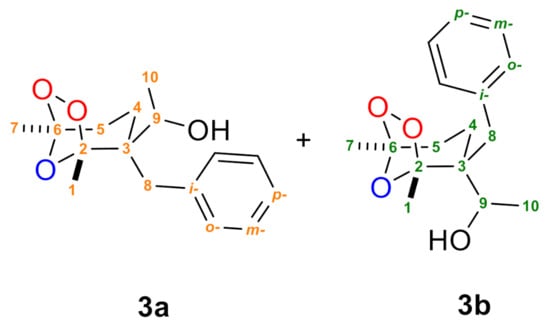
Figure 2.
Ozonides 3a + 3b with numbered atoms.
LiAlH4 (0.055 g, 1.45 mmol) was added to the solution of ozonides 2a + 2b (0.200 g, 0.724 mmol) in (dry) THF (10 mL) with stirring under an argon atmosphere at −22 °C. The reaction mixture was stirred at −22 °C for 3h. After that, an aqueous solution of NaOH (5M, 5 mL) was added with stirring to reaction mixture at −22 °C. Then, the reaction mixture was warmed to room temperature, CHCl3 (30 mL) and H2O (15 mL) were added. The organic layer was separated, and the aqueous layer was extracted with CHCl3 (3 × 15 mL). The combined organic layers were washed with water (5 mL), dried over MgSO4, and then concentrated in vacuo. Ozonides 3a + 3b were isolated by column chromatography on silica gel (EA—hexane, 10:1, v/v). Ozonides 3a + 3b: 0.121 g, 0.43 mmol, 60% yield, colorless oil, Rf = 0.30 (TLC, PE:EA, 5:1).
3a: 1H NMR (300.13 MHz, CDCl3), δ: 1.34 (d, J = 6.5 Hz, 3H, (H10)), 1.56 (s, 3H, (H7)), 1.58 (s, 3H, (H1)), 1.74–2.30 (m, 4H, (H4 ,H5)), 2.34 (br.s. 1H, OH), 2.96 (d, J = 13.6 Hz, 1H, (H8)), 3.10 (d, J = 13.6 Hz, 1H (H8)), 3.62–3.72 (m, 1H, (H9)), 7.28–7.32 (m, 5H, (Ho−, m−. p−)). 13C NMR (75.48 MHz, CDCl3), δ: 19.5 (C1), 19.6(C10), 20.7(C7), 21.6(C4), 31.4(C5), 36.8(C8), 47.8(C3), 69.4(C9), 108.9(C6), 114.2(C2), 126.6(Cp−), 128.2(Cm−), 130.9(Co−), 138.2(Ci−).
3a:1H NMR (300.13 MHz, CDCl3), δ: 1.26 (d, J = 6.5 Hz, 3H (H10)), 1.28–1.38 (m, 1H, (H4)), 1.53 (s, 3H, (H7)), 1.70 (s, 3H, (H1)), 1.74–2.30 (m, 3H, (H4,H5)), 2.33 (br.s. 1H, OH), 2.71 (d, J = 13.6 Hz, 1H, (H8)), 3.05 (d, J = 13.6 Hz, 1H, (H8)), 3.88–3.97 (m, 1H, (H9)), 7.28–7.32 (m, 5H, (Ho−, m−. p−)).13C NMR (75.48 MHz, CDCl3), δ: 19.7(C1), 20.9(C7), 21.1(C10), 24.8(C4), 32.8(C5), 40.4(C8), 46.8(C3), 71.0(C9), 108.6(C6), 113.4(C2), 126.5(Cp−), 128.1(Cm−), 131.1(Co−), 137.6(Ci−).
HRMS (ESI-TOF): m/z [M + Na]+: calculated for [C16H22NaO4]+: 301.1410; found: 301.1409. Anal. Calcd for C16H22O4: C, 69.04; H, 7.97. Found: C, 69.08; H, 8.00. IR (KBr): 3561, 2945, 1722, 1603, 1496, 1453, 1382, 1226, 1141, 1082, 944, 913, 827, 732, 705, 662 cm−1.
4. Conclusions
In this work, we presented a synthesis of the previously unknown compound 3a + 3b (1-(2-benzyl-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octan-2-yl)ethan-1-ol) in 60% isolated yield. The ozonide cycle is surprisingly moderately resistant to LiAlH4.
Supplementary Materials
The following supporting information can be downloaded. Copies of 1H, 13C NMR, HMRS, IR for compounds 3a + 3b.
Author Contributions
Conceptualization, A.O.T.; synthesis of peroxides, P.S.R., writing—review and editing, I.A.Y., project administration, A.O.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Slack, R.D.; Jacobine, A.M.; Posner, G.H. Antimalarial peroxides: Advances in drug discovery and design. MedChemComm 2012, 3, 281–297. [Google Scholar] [CrossRef]
- Pinet, A.; Cojean, S.; Nguyen, L.T.; Vasquez-Ocmin, P.; Maciuk, A.; Loiseau, P.M.; Le Pape, P.; Figadere, B.; Ferrie, L. Anti-protozoal and anti-fungal evaluation of 3,5-disubstituted 1,2-dioxolanes. Bioorg. Med. Chem. Lett. 2021, 47, 128196. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, G.; Giannangelo, C.; De Paoli, A.; Schuh, A.K.; Heimsch, K.C.; Anderson, D.; Brown, T.G.; MacRaild, C.A.; Wu, J.; Wang, X.; et al. Peroxide Antimalarial Drugs Target Redox Homeostasis in Plasmodium falciparum Infected Red Blood Cells. ACS Infect. Dis. 2022, 8, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Y.; Guan, W.; Su, W.; Li, G.; Zhang, S.; Yao, H. Spiral molecules with antimalarial activities: A review. Eur. J. Med. Chem. 2022, 237, 114361. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, C.; Sharma, B.; Mishra, A.; Agarwal, D.; Kannan, D.; Held, J.; Singh, S.; Awasthi, S.K. N-sulfonylpiperidinedispiro-1,2,4,5-tetraoxanes exhibit potent in vitro antiplasmodial activity and in vivo efficacy in mice infected with P. berghei ANKA. Eur. J. Med. Chem. 2022, 244, 114774. [Google Scholar] [CrossRef]
- Li, S.; Xu, W.; Wang, H.; Tang, T.; Ma, J.; Cui, Z.; Shi, H.; Qin, T.; Zhou, H.; Li, L.; et al. Ferroptosis plays an essential role in the antimalarial mechanism of low-dose dihydroartemisinin. Biomed. Pharmacother. 2022, 148, 112742. [Google Scholar] [CrossRef]
- Tiwari, M.K.; Chaudhary, S. Artemisinin Analogues as a Novel Class of Antimalarial Agents: Recent Developments, Current Scenario and Future Perspectives. In Frontiers in Drug Design Discovery; Bentham Science Publishers: Singapore, 2022; Volume 11, pp. 75–115. [Google Scholar]
- Abrams, R.P.; Carroll, W.L.; Woerpel, K.A. Five-Membered Ring Peroxide Selectively Initiates Ferroptosis in Cancer Cells. ACS Chem. Biol. 2016, 11, 1305–1312. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Syroeshkin, M.A.; Levitsky, D.O.; Fleury, F.; Terent’ev, A.O. Cyclic peroxides as promising anticancer agents: In vitro cytotoxicity study of synthetic ozonides and tetraoxanes on human prostate cancer cell lines. Med. Chem. Res. 2017, 26, 170–179. [Google Scholar] [CrossRef]
- Chaudhari, M.B.; Moorthy, S.; Patil, S.; Bisht, G.S.; Mohamed, H.; Basu, S.; Gnanaprakasam, B. Iron-Catalyzed Batch/Continuous Flow C-H Functionalization Module for the Synthesis of Anticancer Peroxides. J. Org. Chem. 2018, 83, 1358–1368. [Google Scholar] [CrossRef]
- Vil’, V.A.; Yaremenko, I.A.; Fomenkov, D.I.; Levitsky, D.O.; Fleury, F.; Terent’ev, A.O. Ion exchange resin-catalyzed synthesis of bridged tetraoxanes possessing in vitro cytotoxicity against HeLa cancer cells. Chem. Heterocycl. Comp. 2020, 56, 722–726. [Google Scholar] [CrossRef]
- Makhmudiyarova, N.N.; Ishmukhametova, I.R.; Dzhemileva, L.U.; Tyumkina, T.V.; D’yakonov, V.A.; Ibragimov, A.G.; Dzhemilev, U.M. Synthesis and anticancer activity novel dimeric azatriperoxides. RSC Adv. 2019, 9, 18923–18929. [Google Scholar] [CrossRef] [PubMed]
- Coghi, P.; Yaremenko, I.A.; Prommana, P.; Wu, J.N.; Zhang, R.L.; Ng, J.P.L.; Belyakova, Y.Y.; Law, B.Y.K.; Radulov, P.S.; Uthaipibull, C.; et al. Antimalarial and Anticancer Activity Evaluation of Bridged Ozonides, Aminoperoxides, and Tetraoxanes. ChemMedChem 2022, 17, e202200328. [Google Scholar] [CrossRef] [PubMed]
- Yaremenko, I.A.; Syromyatnikov, M.Y.; Radulov, P.S.; Belyakova, Y.Y.; Fomenkov, D.I.; Popov, V.N.; Terent’ev, A.O. Cyclic Synthetic Peroxides Inhibit Growth of Entomopathogenic Fungus Ascosphaera apis without Toxic Effect on Bumblebees. Molecules 2020, 25, 1954. [Google Scholar] [CrossRef] [PubMed]
- Yaremenko, I.A.; Radulov, P.S.; Belyakova, Y.Y.; Demina, A.A.; Fomenkov, D.I.; Barsukov, D.V.; Subbotina, I.R.; Fleury, F.; Terent’ev, A.O. Catalyst Development for the Synthesis of Ozonides and Tetraoxanes Under Heterogeneous Conditions: Disclosure of an Unprecedented Class of Fungicides for Agricultural Application. Chem. Eur. J. 2020, 26, 4734–4751. [Google Scholar] [CrossRef]
- Keiser, J.; Utzinger, J.; Tanner, M.; Dong, Y.; Vennerstrom, J.L. The synthetic peroxide OZ78 is effective against Echinostoma caproni and Fasciola hepatica. J. Antimicrob. Chemother. 2006, 58, 1193–1197. [Google Scholar] [CrossRef]
- Zhao, Q.; Vargas, M.; Dong, Y.; Zhou, L.; Wang, X.; Sriraghavan, K.; Keiser, J.; Vennerstrom, J.L. Structure-activity relationship of an ozonide carboxylic acid (OZ78) against Fasciola hepatica. J. Med. Chem. 2010, 53, 4223–4233. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Q.; Vargas, M.; Dong, Y.; Sriraghavan, K.; Keiser, J.; Vennerstrom, J.L. The activity of dispiro peroxides against Fasciola hepatica. Bioorg. Med. Chem. Lett. 2011, 21, 5320–5323. [Google Scholar] [CrossRef]
- Cowan, N.; Yaremenko, I.A.; Krylov, I.B.; Terent’ev, A.O.; Keiser, J. Elucidation of the in vitro and in vivo activities of bridged 1,2,4-trioxolanes, bridged 1,2,4,5-tetraoxanes, tricyclic monoperoxides, silyl peroxides, and hydroxylamine derivatives against Schistosoma mansoni. Bioorg. Med. Chem. 2015, 23, 5175–5181. [Google Scholar] [CrossRef]
- Amado, P.S.M.; Costa, I.C.C.; Paixao, J.A.; Mendes, R.F.; Cortes, S.; Cristiano, M.L.S. Synthesis, Structure and Antileishmanial Evaluation of Endoperoxide-Pyrazole Hybrids. Molecules 2022, 27, 5401. [Google Scholar] [CrossRef]
- Chou, S.; Marousek, G.; Auerochs, S.; Stamminger, T.; Milbradt, J.; Marschall, M. The unique antiviral activity of artesunate is broadly effective against human cytomegaloviruses including therapy-resistant mutants. Antiviral Res. 2011, 92, 364–368. [Google Scholar] [CrossRef]
- Yang, J.J.; Boissier, J.; Chen, J.L.; Yao, H.; Yang, S.; Rognon, A.; Qiao, C. Design, synthesis and biological evaluation of praziquantel and endoperoxide conjugates as antischistosomal agents. Future Med. Chem. 2015, 7, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Ge, X.; Wang, J.; Wei, Z.; Feng, W.H.; Wang, J. Ergosterol peroxide exhibits antiviral and immunomodulatory abilities against porcine deltacoronavirus (PDCoV) via suppression of NF-kappaB and p38/MAPK signaling pathways in vitro. Int. Immunopharmacol. 2021, 93, 107317. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, L.; Yaremenko, I.A.; Capci, A.; Struwe, J.; Tailor, D.; Dheeraj, A.; Hodek, J.; Belyakova, Y.Y.; Radulov, P.S.; Weber, J.; et al. Synthesis and in vitro Study of Artemisinin/Synthetic Peroxide-Based Hybrid Compounds against SARS-CoV-2 and Cancer. ChemMedChem 2022, 17, e202200005. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Hasan, M.H.; Mitra, D.; Bollavarapu, R.; Valente, E.J.; Tandon, R.; Raucher, D.; Hamme, A.T., 2nd. Design, Synthesis, and Preliminary Studies of Spiro-isoxazoline-peroxides against Human Cytomegalovirus and Glioblastoma parallel. J. Org. Chem. 2019, 84, 6992–7006. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, S.D.; Spangler, B.; Gut, J.; Lauterwasser, E.M.; Rosenthal, P.J.; Renslo, A.R. Drug delivery to the malaria parasite using an arterolane-like scaffold. ChemMedChem 2015, 10, 47–51. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Miller, H.; Knox, K.; Kundu, M.; Henrickson, K.J.; Arav-Boger, R. Inhibition of Human Coronaviruses by Antimalarial Peroxides. ACS Infect. Dis. 2021, 7, 1985–1995. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Borisov, D.A.; Yaremenko, I.A. General methods for the preparation of 1,2,4,5-tetraoxanes—Key structures for the development of peroxidic antimalarial agents. Chem. Heterocycl. Comp. 2012, 48, 55–58. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Yaremenko, I.A.; Glinushkin, A.P.; Nikishin, G.I. Synthesis of peroxides from β,δ-triketones under heterogeneous conditions. Russ. J. Org. Chem. 2015, 51, 1681–1687. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Yaremenko, I.A. Stable and Unstable 1,2-Dioxolanes: Origin, Synthesis, and Biological Activities. In Science of Synthesis Knowledge Updates; Georg Thieme Verlag KG: New York, NY, USA, 2020; Volume 2, pp. 277–314. [Google Scholar]
- Radulov, P.S.; Yaremenko, I.A. Application of BF3·Et2O in the synthesis of cyclic organic peroxides (microreview). Chem. Heterocycl. Comp. 2020, 56, 1146–1148. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Radulov, P.S.; Belyakova, Y.Y.; Fomenkov, D.I.; Tsogoeva, S.B.; Terent’ev, A.O. Lewis Acids and Heteropoly Acids in the Synthesis of Organic Peroxides. Pharmaceuticals 2022, 15, 472. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Brautigam, M.; Kleczka, M.; Raabe, A. Synthetic Approaches to Mono- and Bicyclic Perortho-Esters with a Central 1,2,4-Trioxane Ring as the Privileged Lead Structure in Antimalarial and Antitumor-Active Peroxides and Clarification of the Peroxide Relevance. Molecules 2017, 22, 119. [Google Scholar] [CrossRef] [PubMed]
- Ubale, A.S.; Chaudhari, M.B.; Shaikh, M.A.; Gnanaprakasam, B. Manganese-Catalyzed Synthesis of Quaternary Peroxides: Application in Catalytic Deperoxidation and Rearrangement Reactions. J. Org. Chem. 2020, 85, 10488–10503. [Google Scholar] [CrossRef] [PubMed]
- Makhmudiyarova, N.; Ishmukhametova, I.; Dzhemileva, L.; D’yakonov, V.; Ibragimov, A.; Dzhemilev, U. First Example of Catalytic Synthesis of Cyclic S-Containing Di- and Triperoxides. Molecules 2020, 25, 1874. [Google Scholar] [CrossRef] [PubMed]
- Eske, A.; Ecker, S.; Fendinger, C.; Goldfuss, B.; Jonen, M.; Lefarth, J.; Neudorfl, J.M.; Spilles, M.; Griesbeck, A.G. Spirofused and Annulated 1,2,4-Trioxepane-, 1,2,4-Trioxocane-, and 1,2,4-Trioxonane-Cyclohexadienones: Cyclic Peroxides with Unusual Ring Conformation Dynamics. Angew. Chem. Int. Ed. 2018, 57, 13770–13774. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Gomes, G.d.P.; Radulov, P.S.; Belyakova, Y.Y.; Vilikotskiy, A.E.; Vil’, V.A.; Korlyukov, A.A.; Nikishin, G.I.; Alabugin, I.V.; Terent’ev, A.O. Ozone-Free Synthesis of Ozonides: Assembling Bicyclic Structures from 1,5-Diketones and Hydrogen Peroxide. J. Org. Chem. 2018, 83, 4402–4426. [Google Scholar] [CrossRef]
- Shaveta; Mishra, S.; Singh, P. Hybrid molecules: The privileged scaffolds for various pharmaceuticals. Eur. J. Med. Chem. 2016, 124, 500–536. [Google Scholar] [CrossRef]
- Alkhzem, A.H.; Woodman, T.J.; Blagbrough, I.S. Design and synthesis of hybrid compounds as novel drugs and medicines. RSC Adv. 2022, 12, 19470–19484. [Google Scholar] [CrossRef]
- Decker, M. Design of Hybrid Molecules for Drug Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–338. [Google Scholar]
- Russel, A.T. Synthesis by Addition to Alkynes and Alkenes. In Category 5, Compounds with One Saturated Carbon Heteroatom Bond; Georg Thieme Verlag KG: Stuttgart, Germany, 2008. [Google Scholar]
- Story, P.R.; Bishop, C.E.; Burgess, J.R.; Murray, R.W.; Youssefyeh, R.D. Evidence for a new mechanism of ozonolysis. J. Am. Chem. Soc. 1968, 90, 1907–1909. [Google Scholar] [CrossRef]
- Bishop, C.E.; Story, P.R. Mechanisms of ozonolysis. Reductive cleavage of ozonides. J. Am. Chem. Soc. 1968, 90, 1905–1907. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Vil’, V.A.; Demchuk, D.V.; Terent’ev, A.O. Rearrangements of organic peroxides and related processes. Beilstein J. Org. Chem. 2016, 12, 1647–1748. [Google Scholar] [CrossRef]
- Dong, Y.; Wittlin, S.; Sriraghavan, K.; Chollet, J.; Charman, S.A.; Charman, W.N.; Scheurer, C.; Urwyler, H.; Santo Tomas, J.; Snyder, C.; et al. The Structure−Activity Relationship of the Antimalarial Ozonide Arterolane (OZ277). J. Med. Chem. 2009, 53, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Yaremenko, I.A.; Terent’ev, A.O.; Vil’, V.A.; Novikov, R.A.; Chernyshev, V.V.; Tafeenko, V.A.; Levitsky, D.O.; Fleury, F.; Nikishin, G.I. Approach for the preparation of various classes of peroxides based on the reaction of triketones with H2O2: First examples of ozonide rearrangements. Chem. Eur. J. 2014, 20, 10160–10169. [Google Scholar] [CrossRef] [PubMed]
- Saito, I.; Nagata, R.; Yuba, K.; Matsuura, T. Synthesis of α-silyloxyhydroperoxides from the reaction of silyl enol ethers and hydrogen peroxide. Tetrahedron Lett. 1983, 24, 1737–1740. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).