Abstract
A new fluorinated pentaphene derivative has been obtained as a potential precursor for fluorinated polycyclic aromatic hydrocarbons. In this work, 7,7-difluoropentaphen-6(7H)-one was prepared from 1,1-difluoroanthracen-2(1H)-one via the Diels–Alder reaction with o-quinodimethane generated in situ from o-bis(dibromomethyl)benzene. The structure of the newly synthesized compound was confirmed by 1H, 13C, 19F NMR, IR and UV/Vis spectroscopy, as well as high-resolution mass spectrometry.
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are of great interest as semiconducting materials for use in organic electronic and optoelectronic devices, including field-effect transistors, light-emitting diodes and solar cells [1,2]. The design of fluorinated PAHs has been an ongoing task for many research groups: the introduction of a fluorine atom allows one to tune electronic properties, increase the stability of materials and significantly influence their solid-state organization [3,4]. The Diels–Alder reaction has been widely used for PAH synthesis [5,6], and the development of new fluorinated building blocks for cycloaddition is currently of interest. Herein, we report the synthesis of 7,7-difluoropentaphen-6(7H)-one as a potential precursor for fluorinated small molecule PAHs via the Diels–Alder reaction of difluoroanthracenone as the dienophile.
2. Results and Discussion
7,7-Difluoropentaphen-6(7H)-one 1 was synthesized according to our previously developed method based on the procedure by Cava (Scheme 1) [7].
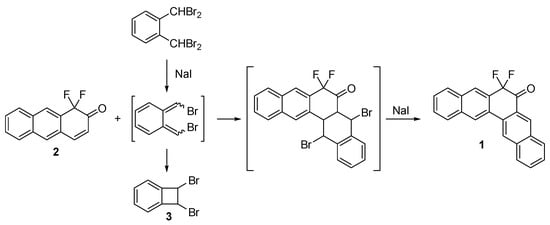
Scheme 1.
Synthesis of 7,7-difluoropentaphen-6(7H)-one 1.
The dibromo-o-quinodimethane generated in situ from o-bis(dibromomethyl)benzene in the presence of NaI acts as a diene and upon trapping by 2 and subsequent aromatization of the primary cycloadduct gives the target pentaphene derivative 1. The workup includes washing the reaction mixture from iodine and DMF with an aqueous solution of sodium thiosulphate and water, respectively, depositing the residue on a short silica column and rinsing it with hexane to eliminate the byproducts generated from diene (mainly dibromobenzocyclobutane 3). Finally, the product is washed off with chloroform and recrystallized to isolate 1 as a yellow solid in 20% yield. The formation of diene cyclization product 3 was confirmed by GCMS (m/z 262 and, after a twofold loss of bromine, 181 and 102) and reported in literature [8].
The structure of 7,7-difluoropentaphen-6(7H)-one 1 was confirmed by means of 1H, 13C, 19F NMR, IR and UV/Vis spectroscopy, as well as high-resolution mass spectrometry. As expected, the 1H NMR spectrum contains four singlets of protons in positions 5, 8, 13 and 14. 13C NMR signals also display highly characteristic C–F coupling constants of 243 Hz (CF2), 26 Hz (CO) and 4.6–22 Hz for other carbon atoms adjacent to the CF2 group (see Supplementary Materials). The spectral data are consistent with those of the previously reported 6,6-difluorotetraphen-5(6H)-one 4, a tetraphenone analogue of 1 (Figure 1) [7]. The addition of a conjugated ring results in a slight redshift of the UV/Vis absorption maxima (295 and 406 nm for 1 vs. 284 and 398 nm for 4) and a shift in the IR spectrum (1704 cm−1 for C=O bond in 1 vs. 1719 cm−1 for 4).
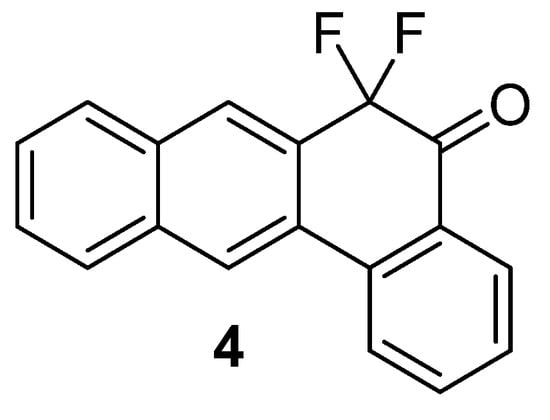
Figure 1.
6,6-Difluorotetraphen-5(6H)-one 4.
We believe that the obtained fluorinated pentaphenone may serve as a precursor for fully aromatic pentaphene derivatives via reductive aromatization, similar to its tetraphenone analogue, giving an angular fluorinated PAH representative.
3. Materials and Methods
1H, 13C and 19F NMR spectra were recorded on Bruker AV-300, AV-400, DRX-500 and AV-600 spectrometers at room temperature; chemical shifts (δ) are given in ppm relative to TMS and CFCl3, respectively. GC/MS spectra were recorded on an Agilent 6890 instrument operating at 70 eV with MSD Agilent 5973. High resolution mass spectra (HRMS) were measured using a Thermo Fisher Scientific Double Focusing System (DFS) Magnetic Sector high resolution mass-spectrometer operating at 70 eV electron ionization and 200 °C ion source temperature. The DFS mass spectrometer was calibrated with respect to the standard lines of perfluorokerosene (PFK) prior to measurements. IR spectra were recorded on a Bruker Vector 22 spectrometer and UV/Vis spectra were recorded on Varian Cary 5000 spectrophotometer (lg(ε) is indicated in brackets). Melting points were recorded on Mettler-Toledo FP81. Column chromatography was carried out on silica gel (40–63 µm) and TLC analysis was performed on silica gel TLC plates. All reactants were of commercial purity and used without further purification, except 1,1-difluoroanthracen-2(1H)-one 2 synthesized according to previously reported method [9] and DMF dried over 3Å molecular sieves.
Synthesis of 7,7-difluoropentaphen-6(7H)-one 1: To a solution of 1,1-difluoroanthracen-2(1H)-one 2 (230 mg, 1.0 mmol) in 7 mL of anhydrous DMF, o-bis(dibromomethyl)benzene (2.65 g, 6 mmol) and finely ground NaI (8.9 g, 60 mmol) were added in 3 equal portions with 30 min interval under vigorous stirring at 80 °C. After the last portion, the reaction mixture was stirred for another 30 min at 80 °C. A solution of Na2S2O3 (17.4 g, 70 mmol of pentahydrate in 25 mL of water) was added and the resulting mixture was extracted with chloroform (4 × 50 mL). The combined organic phase was separated, washed with brine and dried over MgSO4. The solvent was removed under reduced pressure. The residue was percolated on silica with hexane to wash off dihalobenzocyclobutenes. The product was washed off with chloroform, evaporated in vacuum and recrystallized from a hexane–chloroform 1:1 mixture giving the product as a yellow solid. Yield 65 mg (20%). Decomposes without melting. 1H NMR (500 MHz, DMSO-d6), δ: 7.60–7.66 (m, 2H,), 7.69 (t, J = 7.7 Hz, 1H,), 7.75 (t, J = 7.7 Hz, 1H,), 8.07–8.14 (m, 3H,), 8.19 (d, J = 8.0 Hz, 1H), 8.52 (s, 1H), 8.72 (s, 1 H), 8.94 (s, 1H), 8.96 (s, 1H). 13C NMR (500 MHz, DMSO-d6), δ: 109.67 (t, J = 243.2 Hz), 124.19 (s), 125.09 (s), 125.53 (s), 126.40 (t, J = 22.2 Hz), 127.28 (t, J = 5.5 Hz), 127.83 (s), 127.94 (s), 128.35 (t, J = 4.6 Hz), 128.44 (s), 128.53 (s), 128.69 (s), 128.89 (s), 130.08 (s), 130.36 (s), 130.62 (s), 130.90 (s), 132.02 (s), 132.22 (s), 134.69 (s), 136.70 (s), 185.45 (t, J = 25.9 Hz). 19F NMR (282 MHz, DMSO-d6), δ: −99.8 (s). HRMS: Calcd. for (C22H12OF2)+: 330.0851. Found: 330.0845. IR (KBr, cm−1): 3054, 2924, 1704 ν(CO), 1620, 1289, 1115, 747. UV/Vis (CHCl3): λmax = 280 nm (3.72), 295 nm (3.62), 406 nm (1.96).
Supplementary Materials
The following are available online: copies of 1H, 13C, 19F NMR, IR, UV/Vis and HR mass spectra for compound 1.
Author Contributions
Conceptualization, O.T.D. and P.A.Z.; methodology, O.T.D.; software, O.T.D.; validation, O.T.D.; formal analysis, O.T.D.; investigation, O.T.D.; resources, P.A.Z.; data curation, O.T.D.; writing—original draft preparation, O.T.D.; writing—review and editing, P.A.Z.; visualization, O.T.D.; supervision, P.A.Z.; project administration, O.T.D.; funding acquisition, O.T.D. and P.A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Foundation for Basic Research, projects 19-33-60101 and 20-03-00700.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Multi-Access Chemical Research Centre SB RAS at N.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry SB RAS for spectral analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aumaitre, C.; Morin, J. Polycyclic Aromatic Hydrocarbons as Potential Building Blocks for Organic Solar Cells. Chem. Rec. 2019, 19, 1142–1154. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, Y.; Xie, Z.; Zhen, Y.; Hu, W.; Dong, H. Polycyclic Aromatic Hydrocarbon-Based Organic Semiconductors: Ring-Closing Synthesis and Optoelectronic Properties. J. Mater. Chem. C 2022, 10, 2411–2430. [Google Scholar] [CrossRef]
- Ragni, R.; Punzi, A.; Babudri, F.; Farinola, G.M. Organic and Organometallic Fluorinated Materials for Electronics and Optoelectronics: A Survey on Recent Research. Eur. J. Org. Chem. 2018, 2018, 3500–3519. [Google Scholar] [CrossRef]
- Babudri, F.; Farinola, G.M.; Naso, F.; Ragni, R. Fluorinated Organic Materials for Electronic and Optoelectronic Applications: The Role of the Fluorine Atom. Chem. Commun. 2007, 1003–1022. [Google Scholar] [CrossRef] [PubMed]
- Kondratov, I.S.; Tolmachova, N.A.; Haufe, G. Diels-Alder Reaction in the Synthesis of Fluorinated (Hetero)Aromatic Compounds. Eur. J. Org. Chem. 2018, 2018, 3618–3647. [Google Scholar] [CrossRef]
- Dyan, O.T.; Borodkin, G.I.; Zaikin, P.A. The Diels-Alder Reaction for the Synthesis of Polycyclic Aromatic Compounds. Eur. J. Org. Chem. 2019, 2019, 7271–7306. [Google Scholar] [CrossRef]
- Dyan, O.T.; Zaikin, P.A.; Fadeev, D.S.; Gatilov, Y.V.; Borodkin, G.I. 1,1-Difluoronaphthalen-2(1H)-Ones as Building Blocks for Fluorinated Tetraphenes. J. Fluor. Chem. 2018, 210, 88–93. [Google Scholar] [CrossRef]
- Cava, M.P.; Deana, A.A.; Muth, K. Condensed Cyclobutane Aromatic Compounds. VIII. The Mechanism of Formation of 1,2-Dibromobenzocyclobutene; A New Diels-Alder Synthesis. J. Am. Chem. Soc. 1959, 81, 6458–6460. [Google Scholar] [CrossRef]
- Borodkin, G.I.; Zaikin, P.A.; Shubin, V.G. Eco-Friendly Fluorination of Aromatic Compounds with F-TEDA-BF4 Reagent in Water. Chem. Sustain. Dev. 2011, 19, 593–598. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).