(S,S)-1-(Phenyl((1′-4-nitrophenyl-ethyl)amino)methyl)-2-naphthol
Abstract
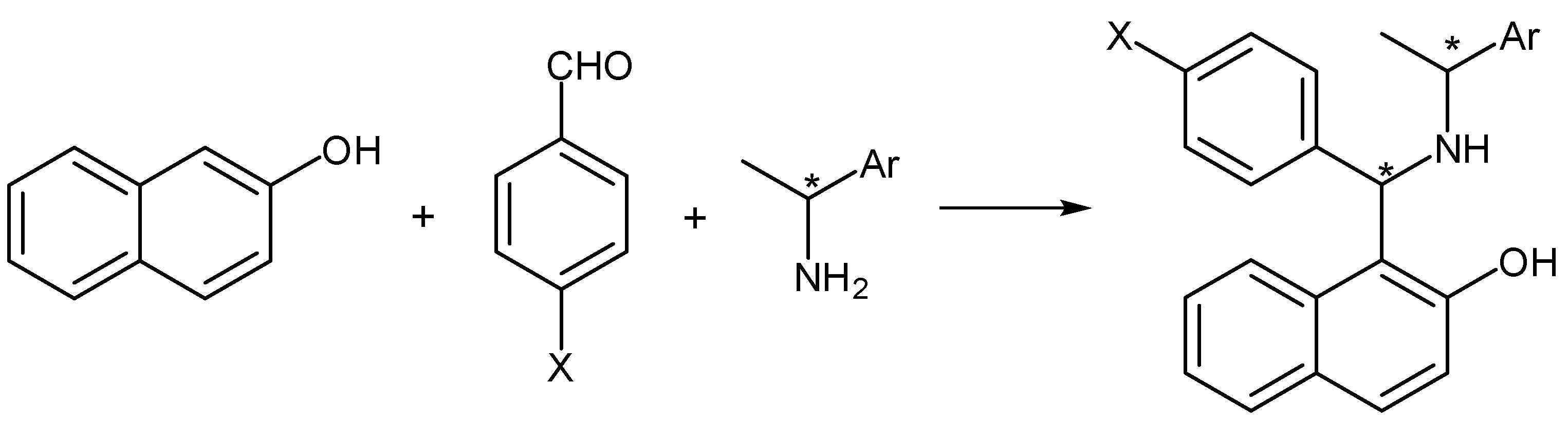
1. Introduction
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardellicchio, C.; Ciccarella, G.F.; Schingaro, E.; Scordari, F. The Betti base: Absolute configuration and routes to a family of related chiral nonracemic bases. Tetrahedron Asymmetry 1998, 9, 3667–3675. [Google Scholar] [CrossRef]
- Cardellicchio, C.; Ciccarella, G.; Naso, F.; Perna, F.; Tortorella, P. Use of readily available chiral compounds related to the Betti base in the enantioselective addition of diethylzinc to aryl aldehydes. Tetrahedron 1999, 55, 14685–14692. [Google Scholar] [CrossRef]
- Naso, F. Mario Betti: A Giant in the Chemistry Scenario of the Twentieth Century. Substantia 2017, 1, 111–121. [Google Scholar]
- Cardellicchio, C.; Capozzi, M.A.M.; Naso, F. The Betti base: The awakening of a sleeping beauty. Tetrahedron Asymmetry 2010, 21, 507–517. [Google Scholar] [CrossRef]
- Iftikhar, R.; Kamran, M.; Iftikhar, A.; Parveen, S.; Naeem, N.; Jamil, N. Recent Advances in the green synthesis of Betti bases and their application: A review. Mol. Divers. 2022. [Google Scholar] [CrossRef] [PubMed]
- Boga, C.; Di Martino, E.; Forlani, L.; Torri, F. Mechanism and diastereoselectivity of the reactions between naphthols and imines. J. Chem. Res. 2001, 2001, 43–45. [Google Scholar] [CrossRef]
- Cimarelli, C.; Mazzanti, A.; Palmieri, G.; Volpini, E. Solvent-Free Asymmetric Aminoalkylation of Electron-Rich Aromatic Compounds: Stereoselective Synthesis of Aminoalkylnaphthols by Crystallization-Induced Asymmetric Transformation. J. Org. Chem. 2001, 66, 4759–4765. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-X.; Zhang, L.-C.; Wang, Q.; Da, C.-S.; Xin, Z.-Q.; Wang, R.; Choi, M.C.K.; Chan, A.S.C. The Application of Chiral Aminonaphthols in the Enantioselective Addition of Diethylzinc to Aryl Aldehydes. Org. Lett. 2001, 3, 2733–2735. [Google Scholar] [CrossRef] [PubMed]
- Cardellicchio, C.; Capozzi, M.A.M.; Alvarez-Larena, A.; Piniella, J.F.; Capitelli, F. Investigation on the weak interactions assembling the crystal structures of Betti bases. CrystEngComm 2012, 14, 3972–3981. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Terraneo, G.; Cardellicchio, C. Structural insights into methyl- or methoxy-substituted 1-(α−aminobenzyl)-2-naphthol structures: The role of C-H···π interactions. Acta Cryst. 2019, C75, 189–195. [Google Scholar] [CrossRef]
- Nishio, M. CH/π hydrogen bonds in crystals. CrystEngComm 2004, 6, 130–158. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Cardellicchio, C. Stereoselection in the Betti reaction of valine methyl esters. Tetrahedron Asymmetry 2017, 28, 1792–1796. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Cardellicchio, C.; Magaletti, A.; Bevilacqua, A.; Perricone, M.; Corbo, M.R. Bioactivity of a Family of Chiral Nonracemic Aminobenzylnaphthols towards Candida albicans. Molecules 2014, 19, 5219–5230. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, M.A.M.; Alvarez-Larena, A.; Piniella Febrer, J.F.; Cardellicchio, C. A combined structural and computational investigation of aminobenzylnaphthol compounds derived from the Betti reaction using valine methyl ester. New J. Chem. 2021, 45, 20735–20742. [Google Scholar] [CrossRef]

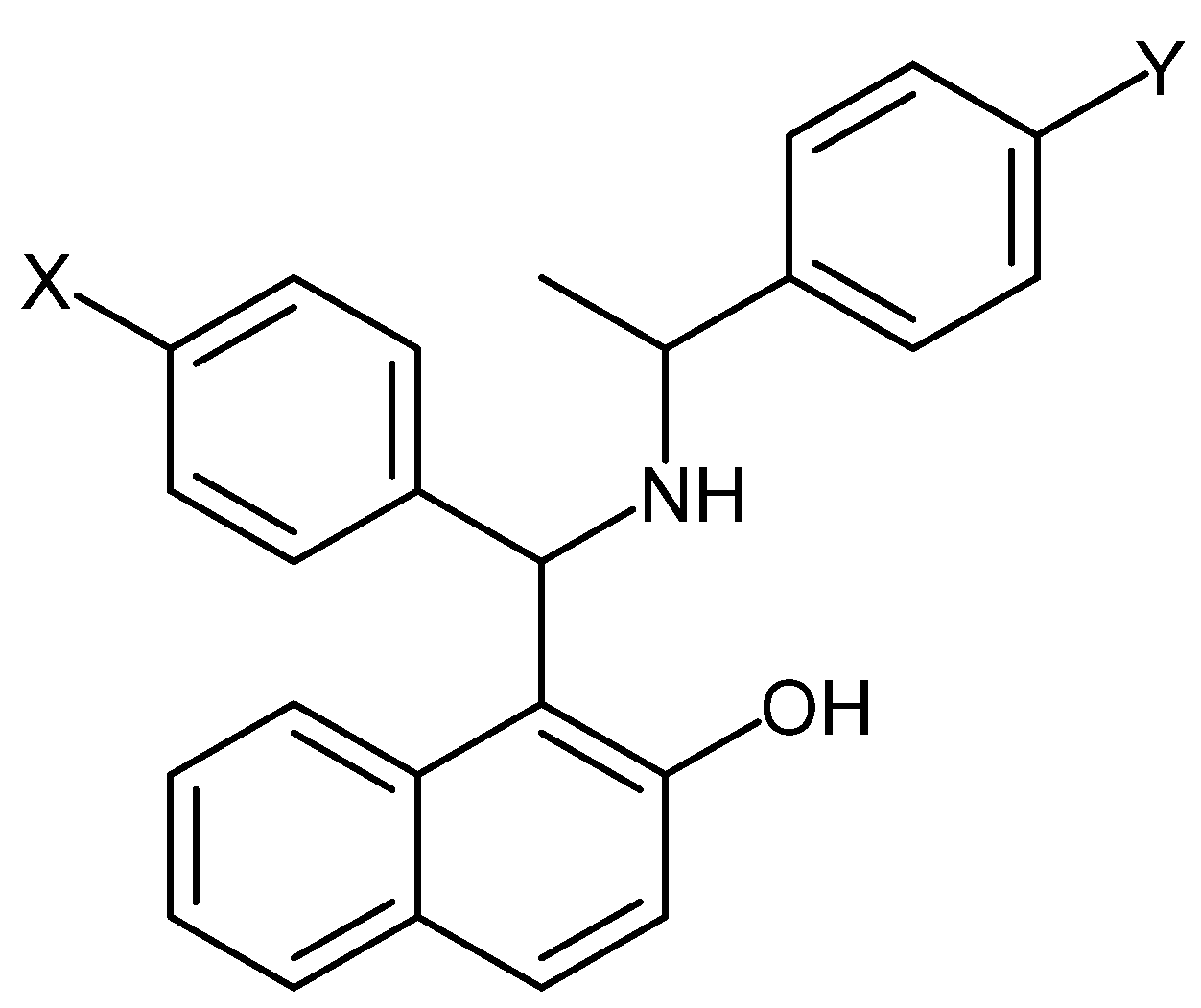
| X | Y | (S,S) 1 | (R,S) 2 |
|---|---|---|---|
| H | H | 5.46 | 5.87 |
| F | H | 5.49 | 5.61 |
| Cl | H | 5.41 | 5.81 |
| Br | H | 5.40 | 5.81 |
| Me | H | 5.43 | 5.84 |
| MeO | H | 5.41 | 5.82 |
| H | MeO | 5.46 | 5.85 |
| H | NO2 | 5.37 | 5.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capozzi, M.A.M.; Cardellicchio, C. (S,S)-1-(Phenyl((1′-4-nitrophenyl-ethyl)amino)methyl)-2-naphthol. Molbank 2022, 2022, M1522. https://doi.org/10.3390/M1522
Capozzi MAM, Cardellicchio C. (S,S)-1-(Phenyl((1′-4-nitrophenyl-ethyl)amino)methyl)-2-naphthol. Molbank. 2022; 2022(4):M1522. https://doi.org/10.3390/M1522
Chicago/Turabian StyleCapozzi, Maria Annunziata M., and Cosimo Cardellicchio. 2022. "(S,S)-1-(Phenyl((1′-4-nitrophenyl-ethyl)amino)methyl)-2-naphthol" Molbank 2022, no. 4: M1522. https://doi.org/10.3390/M1522
APA StyleCapozzi, M. A. M., & Cardellicchio, C. (2022). (S,S)-1-(Phenyl((1′-4-nitrophenyl-ethyl)amino)methyl)-2-naphthol. Molbank, 2022(4), M1522. https://doi.org/10.3390/M1522






