Abstract
4-Methoxyphenethyl (E)-3-(o-tolyl)acrylate (1) was obtained in a good yield by the reaction of 2-methylcinnamic acid, 4-methoxyphenethyl alcohol, 2-methyl-6-nitrobenzoic anhydride, 4-dimethylaminopyridine, and triethylamine at room temperature for 40 min. The structure of 4-methoxyphenethyl (E)-3-(o-tolyl)acrylate (1) was established by FTIR, NMR, and the high resolution of mass spectroscopies. 4-Methoxyphenethyl (E)-3-(o-tolyl)acrylate (1) showed higher α-glucosidase inhibition activity than standard drug acarbose. The molecular docking study exhibited that the title compound 1 had a good affinity for α-glucosidase (PDB ID: 3W37) and formed some interactions with the α-glucosidase active site residue.
1. Introduction
Cinnamic acid esters are found in benzoin balsams [1]. Cinnamic acid esters show biological activities such as antifungal [2], antidiabetic [3], antituberculosis, antimalarial [4], antioxidant [5], antiprotozoal [6], anti-inflammatory [7], and antiproliferative activities [8]. The synthesis of cinnamic acid esters generally involves reactions with thionyl chloride [9], which is one of the chemicals included in the chemical weapon convention (CWC) list [10]. The synthesis of esters can also be carried out using 1,1′-carbonyldiimidazole (CDI) [11] as a coupling agent; however, the resultant intermediate is less reactive than the acyl chloride intermediate [12]. Hydroxybenzotriazole (HOBt) is usually added to the other coupling reagents, such as 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) [13]; however, concerns have been raised due to its explosive properties [14]. On the other hand, 2-methyl-6-nitrobenzoic anhydride (MNBA) showed great promise in the synthesis of carboxylic esters [15]. Herein, we reported the MNBA/DMAP-catalyzed synthesis of 4-methoxyphenethyl (E)-3-(o-tolyl) acrylate (1) and investigated its α-glucosidase inhibition activity. In addition, a molecular docking study was carried out to understand its molecular behavior against the α-glucosidase enzyme.
2. Results and Discussion
2.1. Chemistry
The synthesis of 4-methoxyphenethyl (E)-3-(o-tolyl)acrylate (1) was carried out by the reaction of 2-methylcinnamic acid and 4-methoxyphenethyl alcohol in dichloromethane in the presence of MNBA and DMAP at room temperature (Scheme 1). The crude product was purified by chromatography to yield 4-methoxyphenethyl (E)-3-(o-tolyl)acrylate (1) as a yellowish liquid with a 51% yield. The 1H NMR spectrum of the product confirmed the absence of the carboxylic proton signal of the 4-metylcinnamic acid and exhibited clearly the presence of two triplet signals of the two methylene group protons at 2.95 and 4.37 ppm and a singlet signal of the methoxy group protons at 3.79 ppm for the 4-methoxyphenethyl group. It is strongly supported by the 13C NMR spectrum, which gave signals at 34.42 and 65.36 ppm for the two methylene carbons and a signal at 55.35 ppm for methoxy carbon, and a signal at 167.66 ppm for carbonyl carbon. The IR spectrum showed the presence of carbonyl stretching vibrations at 1707 cm−1 and C-O stretching vibrations at 1163 cm−1 for the ester group. The spectrum also exhibited C-H stretching vibrations at 2954 cm−1, C-H bending vibrations at 1463 cm−1, C-C bending vibrations at 1381 cm−1, and a peak at 1632 cm−1 indicating that the aromatic group conjugated with the alkene group. The high-resolution mass spectrum further supported the product as 4-methoxyphenethyl (E)-3-(o-tolyl)acrylate (1) due to its molecular ion peak at m/z 319.1043 [M + Na]+ in a positive ionization mode.
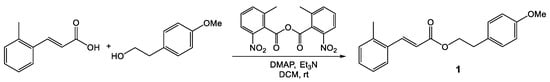
Scheme 1.
Synthesis of 4-methoxyphenethyl (E)-3-(o-tolyl)acrylate (1).
2.2. α-Glucosidase Inhibition Activity
The α-Glucosidase inhibition activity assay of 4-methoxyphenethyl (E)-3-(o-tolyl)acrylate (1) was carried out in vitro to determine its activity in comparison with acarbose as an antidiabetic drug. The assay exhibited that 4-methoxyphenethyl (E)-3-(o-tolyl)acrylate (1) showed inhibition with an IC50 value of 286.39 ± 17.67 µM, which was lower than acarbose with an IC50 value of 475.65 ± 18.88 µM. As a result, the title compound (1) is a better α-glucosidase inhibitor than acarbose.
2.3. Molecular Docking Study
A molecular docking study was carried out to acquire insight into the possible α-glucosidase inhibitory mechanism of 4-methoxyphenethyl (E)-3-(o-tolyl)acrylate (1). The α-Glucosidase complexed with acarbose (PDB ID: 3W37) was chosen as the target for the in silico experiment [16]. Acarbose, as a native ligand, was initially redocked at the active site of α-glucosidase to confirm the docking protocol. The result showed an RMSD value of 1.51 Å, indicating that the protocol could be used for further investigation. An RMSD value lower than 2 Å confirmed that the binding pose of the redocked acarbose was similar to that of the native acarbose [17].
The docking result showed that 4-methoxyphenethyl (E)-3-(o-tolyl)acrylate (1) had a good affinity even though its binding energy was higher than that of acarbose, with values of −7.16 and −8.23 kcal/mol, respectively. 4-Methoxyphenethyl (E)-3-(o-tolyl)acrylate (1) formed two hydrogen bonds: first, the bond between the oxygen atom of the methoxy group and Ala234; and second, the bond between the oxygen atom of the carbonyl group and Arg552 can be seen in Figure 1 and Figure 2. The phenyl ring of the cinnamate skeleton formed π–π stacked hydrophobic and π–anion electrostatic interactions with Phe601: one of the residues at the entrance of the active site, and Asp469, the catalytic residue, respectively. The methyl group attached to the phenyl ring formed π–alkyl hydrophobic interactions with Phe601 and Trp565. The phenyl ring of the phenethyl skeleton formed π–alkyl hydrophobic and π–anion electrostatic interactions with Ala234 and Asp232, respectively. Meanwhile, the methyl group of the methoxy moiety attached to the phenethyl skeleton formed π–alkyl hydrophobic and alkyl interactions with Phe236 and Ala234, respectively. In addition, 4-methoxyphenethyl (E)-3-(o-tolyl)acrylate (1) made contact with several residues on the active site through Van der Waals interactions, including Trp467, His626, Asp357, Trp329, Ile358, Trp432, Met470, Ser474, Asp568, Asn237, and Ile233.
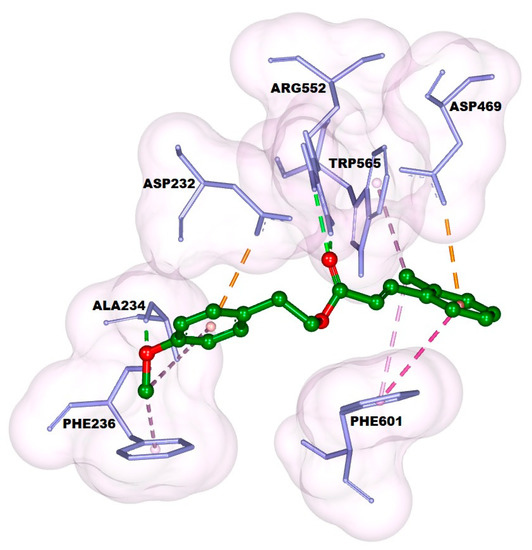
Figure 1.
Docking pose of the title compound (1) against α-glucosidase.
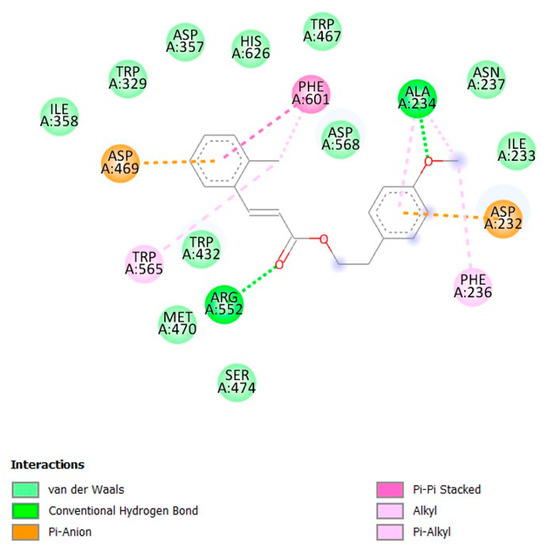
Figure 2.
Ligand interaction diagram of the title compound (1) with α-glucosidase.
3. Materials and Methods
The starting materials and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA), Merck (Rahway, NJ, USA), and Fluka (Charlotte, NC, USA) and were used without further purification. Thin layer chromatography was carried out on Merck 0.20 mm precoated silica gel aluminum plates (Kieselgel 60, F254) and was visualized using a UV lamp operating at 245 nm. Dry column flash chromatography was carried out on Merck 60H [18]. NMR spectra were obtained in CDCl3 on a Jeol JNM-ECS400 spectrometer (400 MHz). A high-resolution mass spectrum was recorded on a Thermo Scientific TSQ Vantage Triple State Quadrupole, and an infrared spectrum was obtained on a Shimadzu 8400S FTIR spectrophotometer.
3.1. Synthesis of 4-Methoxyphenethyl (E)-3-(o-tolyl)acrylate (1)
A solution of 2-methylcinnamic acid (0.26 g; 1.60 mmol), DMAP (0.017 g; 0.14 mmol), and MNBA (0.60 g; 1.74 mmol) in dichloromethane (10 mL) was stirred for 10 min at room temperature. Triethylamine (0.44 mL; 3.16 mmol) and a solution of 4-methoxyphenethyl alcohol (0.22 mL; 1.45 mmol) in dichloromethane (15 mL) were added, and the solution was stirred further for an additional 30 min (the reaction was monitored by TLC with ethyl acetate/n-hexane (1/2) eluant). The solvent was removed under reduced pressure, and the crude product was purified using “dry-column” flash column chromatography with ethyl acetate/n-hexane (1/25) eluant to afford 4-methoxyphenethyl (E)-3-(o-tolyl)acrylate (1) as a yellowish liquid (0.22 g, 51%). 1H NMR (400 MHz, CDCl3) δ 2.42 (s, 3H); 2.95 (t, J = 6.0 Hz, 2H); 3.80 (s, J = 7.6 Hz, 3H); 4.37 (t, J = 6.0 Hz, 2H); 6.33 (d, J = 16.0 Hz, 1H); 6.85 (d, J = 9.2 Hz, 2H); 7.17–7.27 (m, 5H); 7.95 (d, J = 16.0 Hz, 1H); 7.53 (d, J = 7.6 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 19.88, 34.42, 65.36, 55.35, 114.01, 119.16, 126.43, 126.45, 130.02, 130.12, 130.88, 133.44, 137.75, 142.55, 158.39, 167.66. FTIR (KBr) υ (cm−1) 2954 (C-H), 1707 (C=O ester), 1632 (C=C), 1463 (C-H), 1381 (C-C), 1163 (C-O ester). HRESIMS m/z (pos): 319.1043 C19H20O3Na (calcd. 319.1310) (Supplementary Materials).
3.2. α-Glucosidase Inhibitory Activity Assay
The α-Glucosidase inhibition activity assay of the title compound (1) was determined according to the previous method with modifications [19]. Solutions of 4-methoxyphenethyl (E)-3-(o-tolyl)acrylate (1) in DMSO were prepared in some concentrations. Each solution took as much of the 150 μL as possible, which was transferred to a test tube, then added with 210 mL of sodium phosphate buffer pH 6.9 (0.1 M) and 240 µL of the α-glucosidase enzyme (0.4 U/mL) in sodium phosphate buffer pH 6.9 (0.1 M). The test tube was incubated at 37 °C for 10 min, then 300 µL of 4-nitrophenyl-α-D-glucopyranoside (pNPG) (1 mM) in buffer phosphate pH 6.9 (0.1 M) was added to start the reaction. The test tube was further incubated at 37 °C for 20 min, and then 600 µL of Na2CO3 (1.0 M) was added to stop the reaction. The absorbance was measured at a wavelength of 405 nm using a spectrophotometer UV-Vis, and acarbose was used as a standard drug. The enzyme control was prepared by replacing the sample compounds with DMSO. The blank of each sample was arranged by adding the enzyme after stopping the reaction. The reaction was conducted in triplicate. The percentage of α-glucosidase inhibition can be calculated by Equation (1), and the IC50 value was obtained from the linear regression analysis by several test concentrations.
3.3. Molecular Docking Study
The crystallographic structure of sugar beet α-glucosidase complexed with acarbose was taken from the protein data bank (PDB ID: 3W37). The α-glucosidase receptor was prepared using MGLTools 1.5.6 by removing the water molecules, introducing polar hydrogens, and adding Kollman charges [20]. The three-dimensional structure of 4-methoxyphenethyl (E)-3-(o-tolyl)acrylate (1) was minimized using the Merck molecular force field (MMFF49) in the MarvinSketch program. The docking simulation was carried out using the Autodock4.2 software package [21]. The docking pocket was centered at x: 0.052; y: −1.771; and z: −23.298, with xyz-dimensions of 26 × 48 × 26 and spacing of 0.375 Å. The best ligand binding pose was determined using the Lamarckian genetic algorithm with a 200-run GA. The docking result was visualized using BIOVIA Discovery Studio [17].
4. Conclusions
4-Methoxyphenethyl (E)-3-(o-tolyl)acrylate (1) was successfully synthesized from the reaction of 2-methylcinnamic acid and 4-methoxyphenethyl alcohol in the presence of MNBA, DMAP, TEA and showed better α-glucosidase inhibition activity compare with acarbose. Molecular docking exhibited how 4-methoxyphenethyl (E)-3-(o-tolyl)acrylate (1) interacted with the α-glucosidase active site residues through two hydrogen bonds, six hydrophobic interactions and two electrostatic interactions. Cinnamic esters have the potential as lead structures for the optimization of α-glucosidase inhibitors.
Supplementary Materials
The following supporting information can be downloaded online, Figure S1: IR spectrum of the title compound 1; Figure S2: 1H NMR spectrum of the title compound 1; Figure S3: 13C NMR spectrum of the title compound 1; Figure S4: Mass spectrum of the title compound 1.
Author Contributions
Conceptualization, M.S.; Methodology, M.S.; Software, N.P.A.; Validation, A.S.P.; Formal analysis, A.S.P. and S.R.P.; Investigation, E.P., E.Y.R. and Y.A.I.; Data curation, A.S.P. and S.R.P.; Writing—original draft preparation, E.P., E.Y.R. and Y.A.I.; Writing—review and editing, N.P.A. and M.S.; Visualization, N.P.A.; Supervision, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Institut Teknologi Sepuluh Nopember via Penelitian Keilmuan Dana ITS Batch 2 grant number 1641/PKS/ITS/2022.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Acknowledgments
The authors acknowledge the Institut Teknologi Sepuluh Nopember for funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burger, P.; Casale, A.; Kerdudo, A.; Michel, T.; Laville, R.; Chagnaud, F.; Fernandez, X. New insights in the chemical composition of benzoin balsams. Food Chem. 2016, 210, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.C.; Ferreira, A.R.; Silva, D.F.; Lima, E.O.; de Sousa, D.P. Antifungal activity of cinnamic acid and benzoic acid esters against Candida albicans strains. Nat. Prod. Res. 2018, 32, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Anthony, P.C.; Eseyin, O.A.; Attih, E.; Johnson, E.; Ebong, A.; Effiong, A.E. Synthesis of some esters of cinnamic acid and evaluation of their in vitro antidiabetic and antioxidant properties. Trop. J. Pharm. Res. 2022, 21, 131–136. [Google Scholar]
- Bhalodiya, P.C.; Patel, H.N.; Parmar, T.H.; Sangani, C.B.; Rajani, D.P. Novel ester derivative of cinnamates with different long alkoxy chain: Synthesis, mesomorphic properties, biological evaluation. Mol. Cryst. Liq. Cryst. 2021, 724, 1–25. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Torregiani, E.; Bonacucina, G.; Cespi, M.; Palmieri, G.F.; Gabbianelli, R. Antioxidant properties of ester derivatives of cinnamic and hydroxycinnamic acids in Nigella sativa and extra-virgin olive oils-based emulsions. Antioxidants 2022, 11, 194. [Google Scholar] [CrossRef]
- Bernal, F.A.; Kaiser, M.; Wünsch, B.; Schmidt, T.J. Structure−activity relationships of cinnamate ester analogues as potent antiprotozoal agents. ChemMedChem 2020, 15, 68–78. [Google Scholar] [CrossRef]
- Godoy, M.; Rotelli, A.; Pelzer, L.; Tonn, C. Antiinflammatory activity of cinnamic acid esters. Molecules 2000, 5, 547–548. [Google Scholar] [CrossRef]
- Vale, J.; Rodrigues, M.; Lima, M.; Santiago, S.; Domingues, G.; Lima, G.; Andrade Almeida, A.; Oliveira, L.; Bressan, G.; Róbson, R.; et al. Synthesis of cinnamic acid ester derivatives with antiproliferative and antimetastatic activities on murine melanoma cells. Biomed. Pharmacother. 2022, 148, 112689. [Google Scholar] [CrossRef]
- Otero, E.; Robledo, S.; Díaz-Oltra, S.; Carda, M.; Muñoz, D.; Paños, J.; Ve´lez, I.; Cardona Galeano, W. Synthesis and leishmanicidal activity of cinnamic acid esters: Structure-activity relationship. Med. Chem. Res. 2013, 23, 1378–1386. [Google Scholar] [CrossRef]
- Kuitunen, M.-L.; Cecilia Altamirano, J.; Siegenthaler, P.; Hannele Taure, T.; Antero Häkkinen, V.; Sinikka Vanninen, P. Derivatization and rapid GC-MS screening of chlorides relevant to the chemical weapons convention in organic liquid samples. Anal. Methods 2020, 12, 2527–2535. [Google Scholar] [CrossRef]
- Voisin-Chiret, A.S.; Bazin, M.-A.; Lancelot, J.-C.; Rault, S. Synthesis of new L-ascorbic ferulic acid hybrids. Molecules 2007, 12, 2533–2545. [Google Scholar] [CrossRef] [PubMed]
- Aijijiyah, N.P.; Fahmi, M.R.G.; Fatmawati, S.; Santoso, M. Synthesis and molecular docking study of 6-chloropyrazine-2-carboxylic acid derivatives. IOP Conf. Ser. Mater. Sci. Eng. 2020, 833, 012002. [Google Scholar] [CrossRef]
- Brizzi, A.; Trezza, A.; Spiga, O.; Maramai, S.; Scorzelli, F.; Saponara, S.; Fusi, F. 2-Hydroxy-5-(3,5,7-trihydroxy-4-oxo-4H-chromen-2-yl)phenyl (E)-3-(4-hydroxy-3-methoxyphenyl)acrylate: Synthesis, in silico analysis and in vitro pharmacological evaluation. Molbank 2021, 2021, M1258. [Google Scholar] [CrossRef]
- Boyle, M.; Livingstone, K.; Henry, M.C.; Elwood, J.M.L.; Lopez-Fernandez, J.D.; Jamieson, C. Amide bond formation via the rearrangement of nitrile imines derived from N-2-nitrophenyl hydrazonyl bromides. Org. Lett. 2022, 24, 334–338. [Google Scholar] [CrossRef]
- Shiina, I.; Nakata, K. The first asymmetric esterification of free carboxylic acids with racemic alcohols using benzoic anhydrides and tetramisole derivatives: An application to the kinetic resolution of secondary benzylic alcohols. Tetrahedron Lett. 2007, 48, 8314–8317. [Google Scholar] [CrossRef]
- Tagami, T.; Yamashita, K.; Okuyama, M.; Mori, H.; Yao, M.; Kimura, A. Molecular basis for the recognition of long-chain substrates by plant α-glucosidases. J. Biol. Chem. 2013, 288, 19296–19303. [Google Scholar] [CrossRef] [PubMed]
- Jasril, J.; Frimayanti, N.; Nurulita, Y.; Zamri, A.; Ikhtiarudin, I.; Guntur, G. 5-(4-Fluorophenyl)-3-(naphthalen-1-yl)-1-phenyl-1H-pyrazole. Molbank 2021, 2021, M1197. [Google Scholar] [CrossRef]
- Harwood, L.M. “Dry-Column” flash chromatography. Aldrichim. Acta 1985, 18, 25. [Google Scholar]
- Bhatia, A.; Singh, B.; Arora, R.; Arora, S. In vitro evaluation of the α-glucosidase inhibitory potential of methanolic extracts of traditionally used antidiabetic plants. BMC Complem. Altern. Med. 2019, 1, 74. [Google Scholar] [CrossRef]
- Purwanto, B.T.; Siswandono; Kesuma, D.; Widiandani, T.; Siswanto, I. Molecular modeling, admet prediction, synthesis and the cytotoxic activity from the novel N-(4-tert-butylphenylcarbamoyl)benzamide against HeLa. RJC 2021, 14, 1341–1350. [Google Scholar] [CrossRef]
- Santoso, M.; Ong, L.L.; Aijijiyah, N.P.; Wati, F.A.; Azminah, A.; Annuur, R.M.; Fadlan, A.; Judeh, Z.M.A. Synthesis, α-glucosidase inhibition, α-amylase inhibition, and molecular docking studies of 3,3-di(indolyl)indolin-2-ones. Heliyon 2022, 8, e09045. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).