1-(4-Chlorophenyl)-2-methyl-2-phenyl-5-(thiophen-2-yl)-1,2-dihydro-3H-pyrrol-3-one
Abstract
1. Introduction
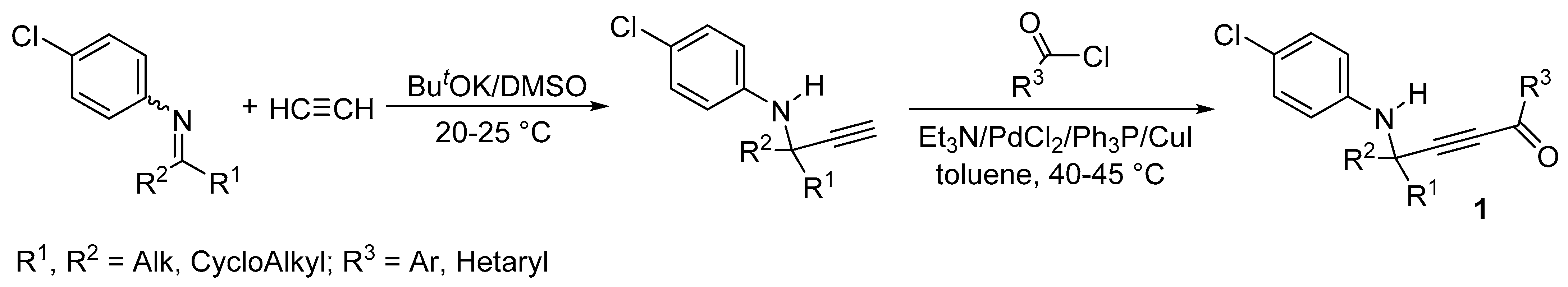
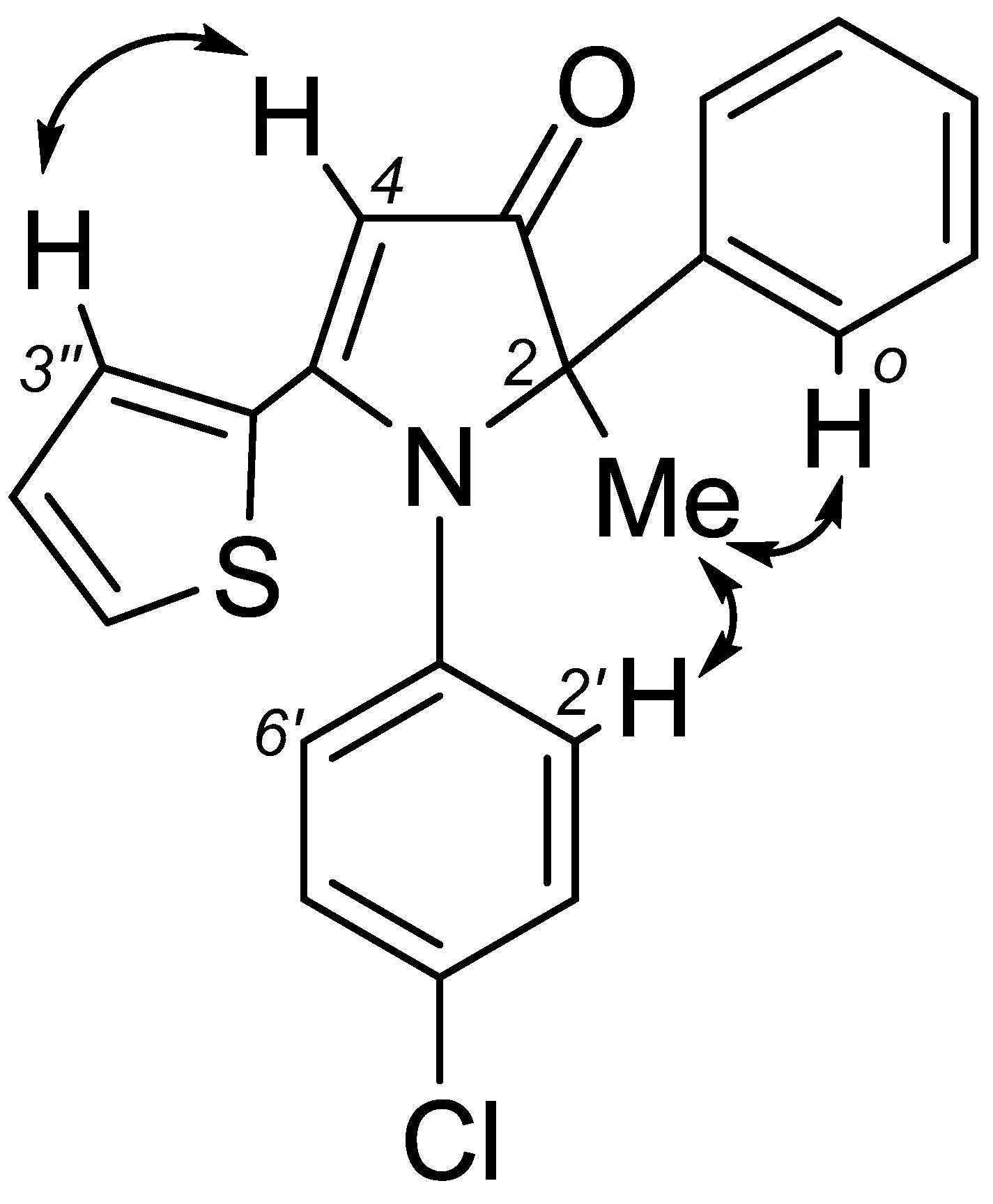
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, M.; Chen, S.; Li, J.; Liu, L. The Biological and Chemical Diversity of Tetramic Acid Compounds from Marine-Derived Microorganisms. Mar. Drugs 2020, 18, 114. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, R.; Bhargava, G. Recent development in the synthesis of pyrrolin-4-ones/pyrrolin-3-ones. J. Heterocycl. Chem. 2020, 57, 4115–4135. [Google Scholar] [CrossRef]
- Karadeolian, A.; Kerr, M.A. Total Synthesis of (+)-Isatisine A. J. Org. Chem. 2010, 75, 6830–6841. [Google Scholar] [CrossRef]
- Murugesan, D.; Mital, A.; Kaiser, M.; Shackleford, D.M.; Morizzi, J.; Katneni, K.; Campbell, M.; Hudson, A.; Charman, S.A.; Yeates, C.; et al. Discovery and Structure–Activity Relationships of Pyrrolone Antimalarials. J. Med. Chem. 2013, 56, 2975–2990. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, D.; Kaiser, M.; White, K.L.; Norval, S.; Riley, J.; Wyatt, P.G.; Charman, S.A.; Read, K.D.; Yeates, C.; Gilbert, I.H. Structure–Activity Relationship Studies of Pyrrolone Antimalarial Agents. ChemMedChem 2013, 8, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.B.; Yu, D.G.; Sun, M.; Zhu, X.X.; Yao, X.J.; Zhou, S.Y.; Chen, J.J.; Gao, K. Ervatamines A–I, Anti-inflammatory Monoterpenoid Indole Alkaloids with Diverse Skeletons from Ervatamia hainanensis. J. Nat. Prod. 2015, 78, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Searcey, M. Duocarmycins–Natures Prodrugs? Curr. Pharm. Des. 2002, 8, 1375–1389. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.B.; Manda, S.; Mupparapu, N.; Battini, N.; Vishwakarma, R.A. Chemistry and Biology of Fascaplysin, a Potent Marine-Derived CDK-4 Inhibitor. Mini-Rev. Med. Chem. 2012, 12, 650–664. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Manjal, S.K.; Rawal, R.K.; Kumar, K. Recent synthetic and medicinal perspectives of tryptanthrin. Bioorg. Med. Chem. 2017, 25, 4533–4552. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.A.; Faheim, A.A.; Elsawy, M.M.; El-Wahab, H.A. Novel flame retardant paint based on Co (II) and Ni (II) metal complexes as new additives for surface coating applications. Appl. Organomet. Chem. 2021, 35, e6070. [Google Scholar] [CrossRef]
- Sobhani, S.; Moghadam, H.H.; Derakhshan, S.R.; Sansano, J.M. Tandem imine formation via auto-hydrogen transfer from alcohols to nitro compounds catalyzed by a nanomagnetically recyclable copper catalyst under solvent-free conditions. RSC Adv. 2021, 11, 19121–19127. [Google Scholar] [CrossRef] [PubMed]
- Gondi, S.R.; Shaik, A.; Westover, K.D. Acid-Catalyzed Synthesis of Isatoic Anhydride-8-Secondary Amides Enables IASA Transformations for Medicinal Chemistry. J. Org. Chem. 2022, 87, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Hamilakis, S.; Tsolomitis, A. An efficient synthesis of 2-amino-3-cyano-2-pyrrolin-4-ones, via the corresponding open chain tautomers (aminoacetylmalononitriles). Tetrahedron Lett. 2003, 44, 3821–3823. [Google Scholar] [CrossRef]
- Gouault, N.; Le Roch, M.; Cornée, C.; David, M.; Uriac, P. Synthesis of Substituted Pyrrolin-4-ones from Amino Acids in Mild Conditions via a Gold-Catalyzed Approach. J. Org. Chem. 2009, 74, 5614–5617. Available online: https://pubs.acs.org/action/showCitFormats?doi=10.1021%2Fjo900693a&href=/doi/10.1021%2Fjo900693a (accessed on 1 November 2022). [CrossRef] [PubMed]
- Spina, R.; Colacino, E.; Gabriele, B.; Salerno, G.; Martinez, J.; Lamaty, F. Synthesis of Pyrrolin-4-ones by Pt-Catalyzed Cycloisomerization in PEG under Microwaves. J. Org. Chem. 2013, 78, 2698–2702. [Google Scholar] [CrossRef] [PubMed]
- Grošelj, U.; Ciber, L.; Gnidovec, J.; Testen, Ž.; Požgan, F.; Štefane, B.; Tavčar, G.; Svete, J.; Ričko, S. Synthesis of Spiro-Δ2-Pyrrolin-4-One Pseudo Enantiomers via an Organocatalyzed Sulfa-Michael/Aldol Domino Sequence. Adv. Synth. Catal. 2019, 361, 5118–5126. [Google Scholar] [CrossRef]
- Schmidt, E.Y.; Bidusenko, I.A.; Protsuk, N.I.; Demyanov, Y.V.; Ushakov, I.A.; Trofimov, B.A. Superbase-promoted addition of acetylene gas to the C=N bond. Eur. J. Org. Chem. 2019, 2019, 5875–5881. [Google Scholar] [CrossRef]
- Volkov, P.A.; Khrapova, K.O.; Bidusenko, I.A.; Telezhkin, A.A.; Schmidt, E.Y.; Albanov, A.I.; Trofimov, B.A. Chemoselective cross-coupling of terminal propargylamines with (het)aroyl chlorides: Synthesis of β-aminoacetylene ketones bearing aromatic and heteroaromatic substituents. Russ. Chem. Bull. 2022, 71, 1514–1518. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkov, P.A.; Khrapova, K.O.; Telezhkin, A.A.; Bidusenko, I.A.; Albanov, A.I.; Trofimov, B.A. 1-(4-Chlorophenyl)-2-methyl-2-phenyl-5-(thiophen-2-yl)-1,2-dihydro-3H-pyrrol-3-one. Molbank 2022, 2022, M1520. https://doi.org/10.3390/M1520
Volkov PA, Khrapova KO, Telezhkin AA, Bidusenko IA, Albanov AI, Trofimov BA. 1-(4-Chlorophenyl)-2-methyl-2-phenyl-5-(thiophen-2-yl)-1,2-dihydro-3H-pyrrol-3-one. Molbank. 2022; 2022(4):M1520. https://doi.org/10.3390/M1520
Chicago/Turabian StyleVolkov, Pavel A., Kseniya O. Khrapova, Anton A. Telezhkin, Ivan A. Bidusenko, Alexander I. Albanov, and Boris A. Trofimov. 2022. "1-(4-Chlorophenyl)-2-methyl-2-phenyl-5-(thiophen-2-yl)-1,2-dihydro-3H-pyrrol-3-one" Molbank 2022, no. 4: M1520. https://doi.org/10.3390/M1520
APA StyleVolkov, P. A., Khrapova, K. O., Telezhkin, A. A., Bidusenko, I. A., Albanov, A. I., & Trofimov, B. A. (2022). 1-(4-Chlorophenyl)-2-methyl-2-phenyl-5-(thiophen-2-yl)-1,2-dihydro-3H-pyrrol-3-one. Molbank, 2022(4), M1520. https://doi.org/10.3390/M1520







