3-(3-Bromophenyl)-7-acetoxycoumarin
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General
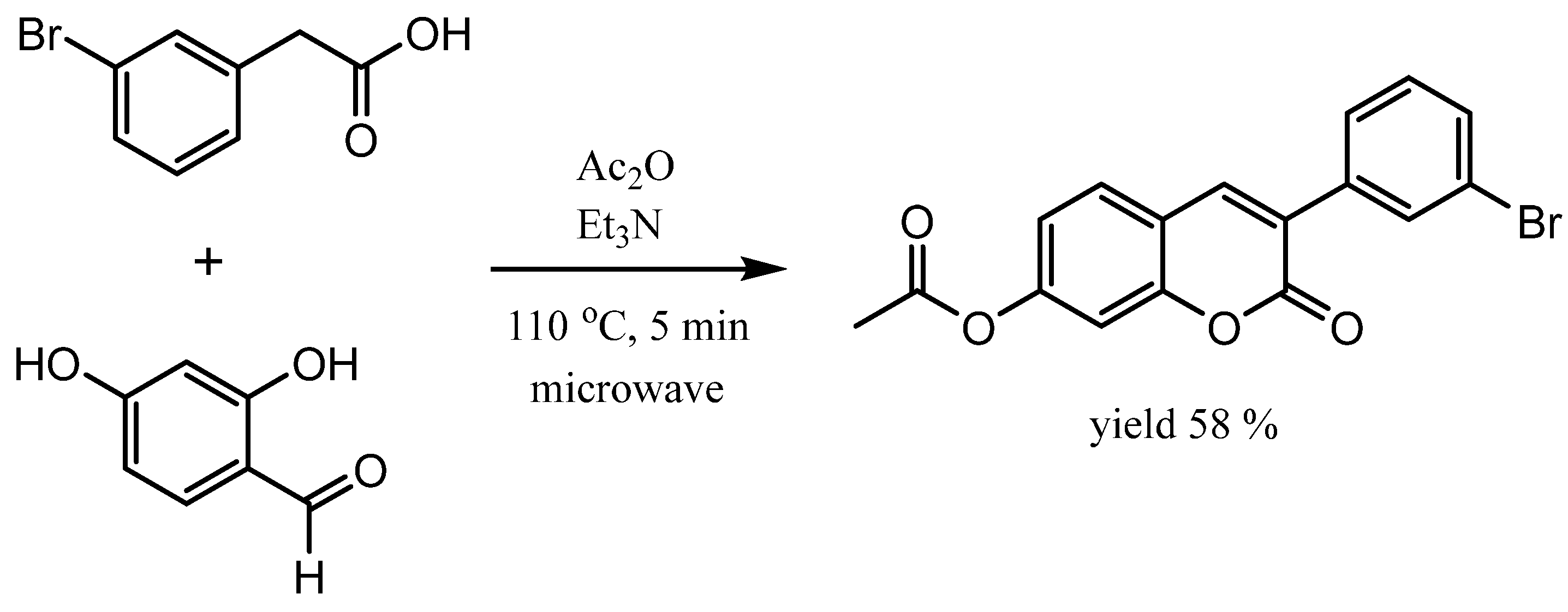
4.2. Synthesis of 3-(3-Bromophenyl)-7-acetoxycoumarin
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouhaoui, A.; Eddahmi, M.; Dib, M.; Khouili, M.; Aires, A.; Catto, M.; Bouissane, L. Synthesis and biological properties of coumarin derivatives. A review. ChemistrySelect 2021, 6, 5848–5870. [Google Scholar] [CrossRef]
- Carneiro, A.; João, M.; Uriarte, E.; Lourdes, S. Trending topics on coumarin and its derivatives in 2020. Molecules 2021, 26, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Uriarte, E.; Santana, L. 3-Phenylcoumarins as a Privileged Scaffold in Medicinal Chemistry: The Landmarks of the Past Decade. Molecules 2021, 26, 6755. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Mohi-ud-din, R.; Sabreen, S.; Banday, N.; Maqbool, M. Coumarin Derivatives as Potential Anti-inflammatory Agents for Drug Development. In Frontiers in Natural Product Chemistry Volume 8, 1st ed.; Atta-Ur-Rahman, F.R.S., Ed.; Bentham Science Publisher: Sharjah, United Arab Emirates, 2021; Volume 8, pp. 213–238. [Google Scholar] [CrossRef]
- Timonen, J.M.; Nieminen, R.M.; Sareila, O.; Goulas, A.; Moilanen, L.J.; Haukka, M.; Vainiotalo, P.; Moilanen, E.; Aulaskari, P.H. Synthesis and anti-inflammatory effects of a series of novel 7-hydroxycoumarin derivatives. Eur. J. Med. Chem. 2021, 46, 3845–3850. [Google Scholar] [CrossRef] [PubMed]
- Raunio, H.; Pentikäinen, O.; Juvonen, R.O. Coumarin-Based Profluorescent and Fluorescent Substrates for Determining Xenobiotic-Metabolizing Enzyme Activities In Vitro. Int. J. Mol. Sci. 2020, 21, 4708. [Google Scholar] [CrossRef] [PubMed]
- Elgemeie, G.H.; Reham, A.M. Microwave synthesis of fluorescent and luminescent dyes (1990–2017). J. Mol. Struct. 2018, 1173, 707–742. [Google Scholar] [CrossRef]
- Enríquez-Palacios, E.; Arbeloa, T.; Bañuelos, J.; Bautista-Hernández, C.I.; Becerra-González, J.G.; López-Arbeloa, I.; Peña-Cabrera, E. Ready Access to Molecular Rotors Based on Boron Dipyrromethene Dyes-Coumarin Dyads Featuring Broadband Absorption. Molecules 2020, 25, 781. [Google Scholar] [CrossRef] [PubMed]
- Tianzhi, Y.; Zeyang, Z.; Yanjun, B.; Yuling, Z.; Xiaoxiao, L.; Hui, Z. Investigation of novel carbazole-functionalized coumarin derivatives as organic luminescent materials. Dyes Pigments 2017, 147, 260–269. [Google Scholar] [CrossRef]
- Sovari, S.N.; Vojnovic, S.; Bogojevic, S.S.; Crochet, A.; Pavic, A.; Nikodinovic-Runic, J.; Zobi, F. Design, synthesis and in vivo evaluation of 3-arylcoumarin derivatives of rhenium(I) tricarbonyl complexes as potent antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 2020, 205, 112533. [Google Scholar] [CrossRef] [PubMed]
- Timonen, J.M.; Vuolteenaho, K.; Leppänen, T.; Nieminen, R.M.; Aulaskari, P.; Jänis, J.; Vainiotalo, P.; Moilanen, E. Synthesis of Novel Anti-inflammatory Psoralen Derivatives—Structures with Distinct Anti-Inflammatory Activities. J. Heterocycl. Chem. 2018, 55, 2590–2597. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turhanen, P.A.; Nousiainen, L.P.; Timonen, J.M. 3-(3-Bromophenyl)-7-acetoxycoumarin. Molbank 2022, 2022, M1513. https://doi.org/10.3390/M1513
Turhanen PA, Nousiainen LP, Timonen JM. 3-(3-Bromophenyl)-7-acetoxycoumarin. Molbank. 2022; 2022(4):M1513. https://doi.org/10.3390/M1513
Chicago/Turabian StyleTurhanen, Petri A., Liisa P. Nousiainen, and Juri M. Timonen. 2022. "3-(3-Bromophenyl)-7-acetoxycoumarin" Molbank 2022, no. 4: M1513. https://doi.org/10.3390/M1513
APA StyleTurhanen, P. A., Nousiainen, L. P., & Timonen, J. M. (2022). 3-(3-Bromophenyl)-7-acetoxycoumarin. Molbank, 2022(4), M1513. https://doi.org/10.3390/M1513







