N,N′-4,5-Dimethoxy-1,2-phenylenebis(salicylideneiminato)nickel(II)
Abstract
1. Introduction
2. Results and Discussion
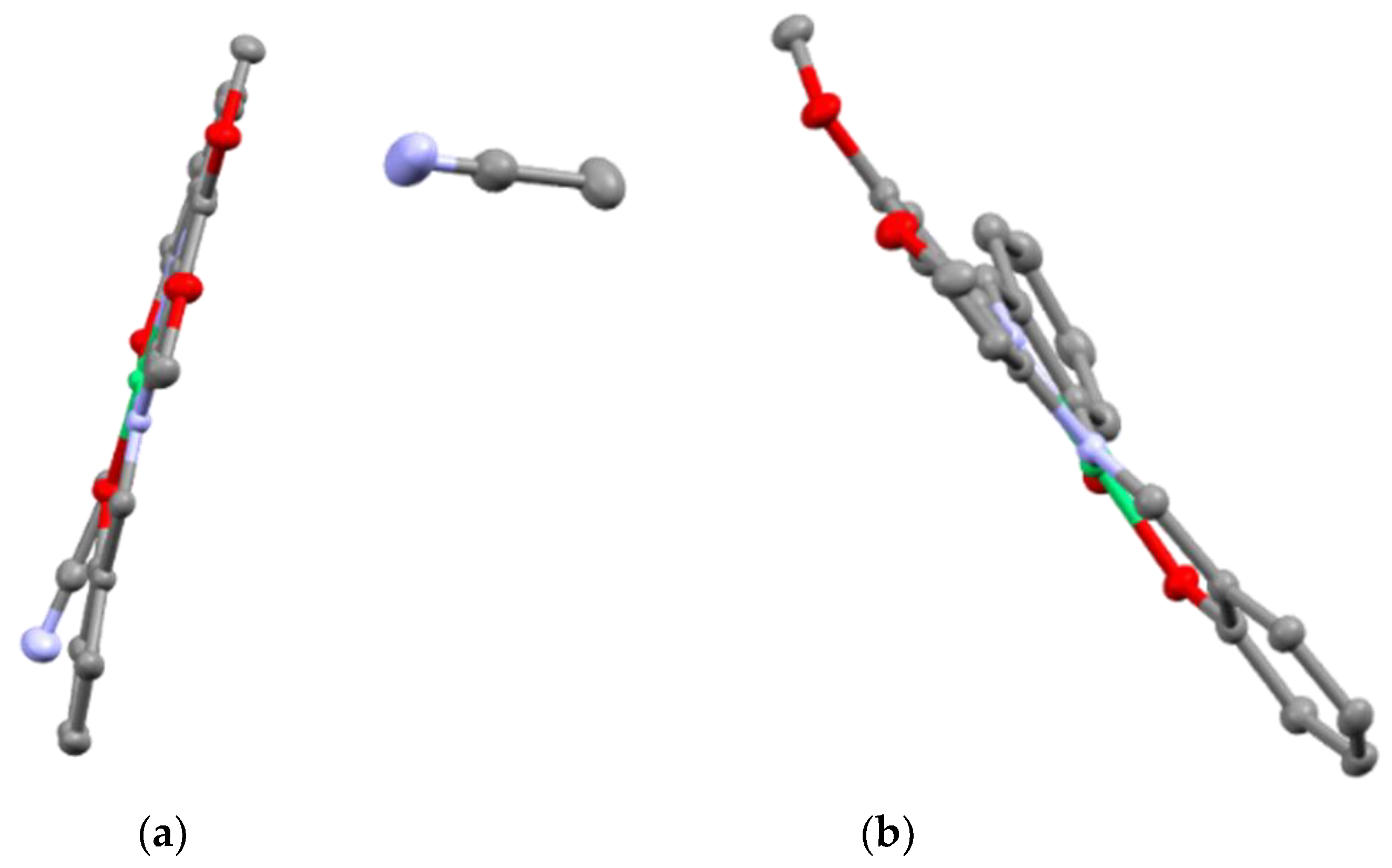
2.1. X-ray Structural Analysis
2.2. Infrared Spectroscopic and Nuclear Magnetic Resonance Studies
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chepurnaya, I.A.; Karushev, M.P.; Alekseeva, E.V.; Lukyanov, D.A.; Levin, O.V. Redox-conducting polymers based on metal-salen complexes for energy storage applications. Pure Appl. Chem. 2020, 92, 1239–1258. [Google Scholar] [CrossRef]
- Freire, C.; Nunes, M.; Pereira, C.; Fernandes, D.M.; Peixoto, A.F.; Rocha, M. Metallo(salen)complexes as versatile building blocks for the fabrication of molecular materials and devices with tuned properties. Coord. Chem. Rev. 2019, 394, 104–134. [Google Scholar] [CrossRef]
- Beletskii, E.V.; Fedorova, A.A.; Lukyanov, D.A.; Kalnin, A.Y.; Ershov, V.A.; Danilov, S.E.; Spiridonova, D.V.; Alekseeva, E.V.; Levin, O.V. Switchable resistance conducting-polymer layer for Li-ion battery overcharge protection. J. Power Sources 2021, 490, 229548. [Google Scholar] [CrossRef]
- Besedina, M.A.; Smirnova, E.A.; Poturai, D.O.; Karushev, M.P. The activity of monomeric and polymeric nickel complexes with Salen-type ligands as photosensitive materials for electrochemical solar cells. Russ. Chem. Bull. 2021, 70, 107–112. [Google Scholar] [CrossRef]
- Smirnova, E.A.; Timonov, A.M. A novel functional material for the electrochemical reduction of chlorinated organic compounds. Russ. Chem. Bull. 2021, 70, 1618–1621. [Google Scholar] [CrossRef]
- Polozhentseva, Y.A.; Novozhilova, M.V.; Chepurnaya, I.A.; Karushev, M.P. Polymeric Complexes of Nickel with Salen-Type Ligands as Multifunctional Components of Lithium Ion Battery Cathodes. Tech. Phys. Lett. 2021, 47, 83–87. [Google Scholar] [CrossRef]
- Dmitrieva, E.; Rosenkranz, M.; Danilova, J.S.; Smirnova, E.A.; Karushev, M.P.; Chepurnaya, I.A.; Timonov, A.M. Radical formation in polymeric nickel complexes with N2O2 Schiff base ligands: An in situ ESR and UV-vis-NIR spectroelectrochemical study. Electrochim. Acta 2018, 283, 1742–1752. [Google Scholar] [CrossRef]
- Dmitrieva, E.A.; Chepurnaya, I.A.; Karushev, M.P.; Timonov, A.M. The Nature of Charge Carriers in Polymeric Complexes of Nickel with Schiff Bases Containing Electron-Withdrawing Substituents. Russ. J. Electrochem. 2019, 55, 1039–1046. [Google Scholar] [CrossRef]
- Danilova, Y.S.; Bykov, V.A.; Spiridonova, D.V.; Novozhilova, M.V.; Polozhentseva, Y.A.; Karushev, M.P.; Timonov, A.M. Synthesis and Structure of Nickel(II) Complex with Methyl-Substituted N2O2 Tetradentate Shiff Base. Russ. J. Gen. Chem. 2021, 91, 747–749. [Google Scholar] [CrossRef]
- Karushev, M.P.; Khoroshilova, O.V.; Kurchavov, D.S.; Novozhilova, M.V.; Timonov, A.M. Supramolecular Associates of Nickel(II) Complexes with Nitro-Substituted Tetradentate Schiff Bases. Russ. J. Gen. Chem. 2020, 90, 444–447. [Google Scholar] [CrossRef]
- Łępicka, K.; Pieta, P.; Francius, G.; Walcarius, A.; Kutner, W. Structure-reactivity requirements with respect to nickel-salen based polymers for enhanced electrochemical stability. Electrochim. Acta 2019, 315, 75–83. [Google Scholar] [CrossRef]
- Schley, M.; Lönnecke, P.; Hey-Hawkins, E. Monometallic and heterobimetallic complexes derived from salen-type ligands. J. Organomet. Chem. 2009, 694, 2480–2487. [Google Scholar] [CrossRef]
- Rosaand, D.T.; Coucouvanis, D. Crown-Ether-Functionalized Nickel Salicylaldimine Complexes. Structural Characterization of Their Potassium, Cesium, and Hexylammonium Derivatives and Their Use in the Transport of Amino Acids. Inorg. Chem. 1998, 37, 2328–2329. [Google Scholar] [CrossRef]
- Pike, J.D.; Rosa, D.T.; Coucouvanis, D. Lipophilic Metal-Salicylideneimine-Crown Ether Hybrids-Ditopic Carriers in the Facilitated Transport of Amphiphilic Molecules Across Bulk Liquid Membranes. Eur. J. Inorg. Chem. 2001, 2001, 761–777. [Google Scholar] [CrossRef]
- Chaudhry, M.T.; Ota, S.; Lelj, F.; MacLachlan, M.J. Breathing Room: Restoring Free Rotation in a Schiff-Base Macrocycle through Endoperoxide Formation. Org. Lett. 2021, 23, 9538–9542. [Google Scholar] [CrossRef]
- Leung, A.C.W.; Chong, J.H.; Patrick, B.O.; MacLachlan, M.J. Poly(salphenyleneethynylene)s: A New Class of Soluble, Conjugated, Metal-Containing Polymers. Macromolecules 2003, 36, 5051–5054. [Google Scholar] [CrossRef]
- Akine, S.; Miyashita, M.; Piao, S.; Nabeshima, T. Perfect encapsulation of a guanidinium ion in a helical trinickel(II) metallocryptand for efficient regulation of the helix inversion rate. Inorg. Chem. Front. 2014, 1, 53–57. [Google Scholar] [CrossRef]
- Akine, S.; Miyashita, M.; Nabeshima, T. Enhancement of Alkali Metal Ion Recognition by Metalation of a Tris(saloph) Cryptand Having Benzene Rings at the Bridgeheads. Inorg. Chem. 2021, 60, 12961–12971. [Google Scholar] [CrossRef]
- Rotthaus, O.; Jarjayes, O.; Philouze, C.; del Valle, C.P.; Thomas, F. One-electron oxidized nickel(II) complexes of bis and tetra(salicylidene) phenylenediamine Schiff bases: From monoradical to interacting Ni(III) ions. Dalton Trans. 2009, 10, 1792–1800. [Google Scholar] [CrossRef]
- Danilova, J.S.; Avdoshenko, S.M.; Karushev, M.P.; Timonov, A.M.; Dmitrieva, E. Infrared spectroscopic study of nickel complexes with salen-type ligands and their polymers. J. Mol. Struct. 2021, 1241, 130668. [Google Scholar] [CrossRef]
- Fan, K.W.; Peterson, M.B.; Ellersdorfer, P.; Granville, A.M. Expanding the aqueous-based redox-facilitated self-polymerization chemistry of catecholamines to 5,6-dihydroxy-1H-benzimidazole and its 2-substituted derivatives. RSC Adv. 2016, 6, 25203–25214. [Google Scholar] [CrossRef]
- Holm, R.H.; Everett, G.W.; Chakravorty, A. Metal Complexes of Schiff Bases and β-Ketoamines. Prog. Inorg. Chem. 1966, 7, 83–214. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]


| Parameter | Value | |
|---|---|---|
| Structure | a | b |
| Empirical formula | C24H21NiN3O4 | C24H21NiN3O4 |
| M | 474.15 | 474.15 |
| Temperature/K | 100(2) | 100(2) |
| Crystal system | monoclinic | trigonal |
| Space group | P21/n | R3c |
| a/Å | 7.95760(10) | 38.7229(7) |
| b/Å | 12.98650(10) | 38.7229(7) |
| c/Å | 20.1006(2) | 7.2027(2) |
| α/° | 90 | 90 |
| β/° | 99.9830(10) | 90 |
| γ/° | 90 | 120 |
| Volume/Å3 | 2045.77(4) | 9353.2(4) |
| Z | 4 | 18 |
| ρ calc/g/cm3 | 1.539 | 1.515 |
| μ/mm−1 | 1.694 | 1.668 |
| F(000) | 984.0 | 4428.0 |
| Crystal size/mm | 0.1 × 0.08 × 0.05 | 0.16 × 0.04 × 0.02 |
| Radiation/λ/Å | CuKα1.54184) | CuKα (1.54184) |
| 2Θ range for data collection/° | 8.142–138.212 | 4.564 to 139.994 |
| Index ranges | −9 ≤ h ≤ 9, −10 ≤ k ≤ 15, −24 ≤ l ≤ 24 | −46 ≤ h ≤ 32, −47 ≤ k ≤ 41, −8 ≤ l ≤ 8 |
| Reflections collected | 11517 | 18976 |
| Independent reflections | 3812 [Rint = 0.0261, Rsigma = 0.0245] | 3872 [Rint = 0.0453, Rsigma = 0.0347] |
| GOOF by F2 | 1.074 | 1.039 |
| R factors [I > = 2σ (I)] | R1 = 0.0278, wR2 = 0.0721 | R1 = 0.0310, wR2 = 0.0773 |
| R factors [all reflections] | R1 = 0.0304, wR2 = 0.0737 | R1 = 0.0325, wR2 = 0.0783 |
| Largest diff. peak/hole, e Å−3 | 0.23/−0.29 | 0.29/−0.28 |
| IR band, cm−1 | Assignment |
|---|---|
| ligand core molecule | |
| 1585–1608 | C = N str in azomethine and C –C str in phenolic ring |
| 1518 | C–C str in phenolic ring |
| 1450–1465 | C–O, C=N and C–C str in phenolic and six-membered chelate ring |
| 1367 | C = N in six-membered chelate ring and C –C str in phenolic ring |
| 1333 | C –O and C –C str in phenolic ring |
| 1244 | C –C str in phenolic and six-membered chelate ring |
| 1109–1189 | C –N str in five-membered chelate ring |
| 729–753 | ring vib and C –H out-of-plane def in phenolic ring |
| metal chelate | |
| 594 | asym O –Ni –O str |
| 459 | asym N –Ni –N str |
| substituents in phenolate moieties | |
| 2831 | sym and asym C –H str in –O–CH3 |
| 1090–1100 | sym C–O–C str in phenyl–O–CH3 |
| diimine backbone | |
| 3042–3012 | sym and asym C–H str in o –phenylene |
| 1146 | C–H in-plane def in o –phenylene |
| 753 | ring vib and C –H out-of-plane def in o -phenylene |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smirnova, E.; Baichurin, R.; Viktorov, N.; Spiridonova, D.; Timonov, A.; Karushev, M. N,N′-4,5-Dimethoxy-1,2-phenylenebis(salicylideneiminato)nickel(II). Molbank 2022, 2022, M1512. https://doi.org/10.3390/M1512
Smirnova E, Baichurin R, Viktorov N, Spiridonova D, Timonov A, Karushev M. N,N′-4,5-Dimethoxy-1,2-phenylenebis(salicylideneiminato)nickel(II). Molbank. 2022; 2022(4):M1512. https://doi.org/10.3390/M1512
Chicago/Turabian StyleSmirnova, Evgenia, Ruslan Baichurin, Nikolai Viktorov, Dar’ya Spiridonova, Alexander Timonov, and Mikhail Karushev. 2022. "N,N′-4,5-Dimethoxy-1,2-phenylenebis(salicylideneiminato)nickel(II)" Molbank 2022, no. 4: M1512. https://doi.org/10.3390/M1512
APA StyleSmirnova, E., Baichurin, R., Viktorov, N., Spiridonova, D., Timonov, A., & Karushev, M. (2022). N,N′-4,5-Dimethoxy-1,2-phenylenebis(salicylideneiminato)nickel(II). Molbank, 2022(4), M1512. https://doi.org/10.3390/M1512






