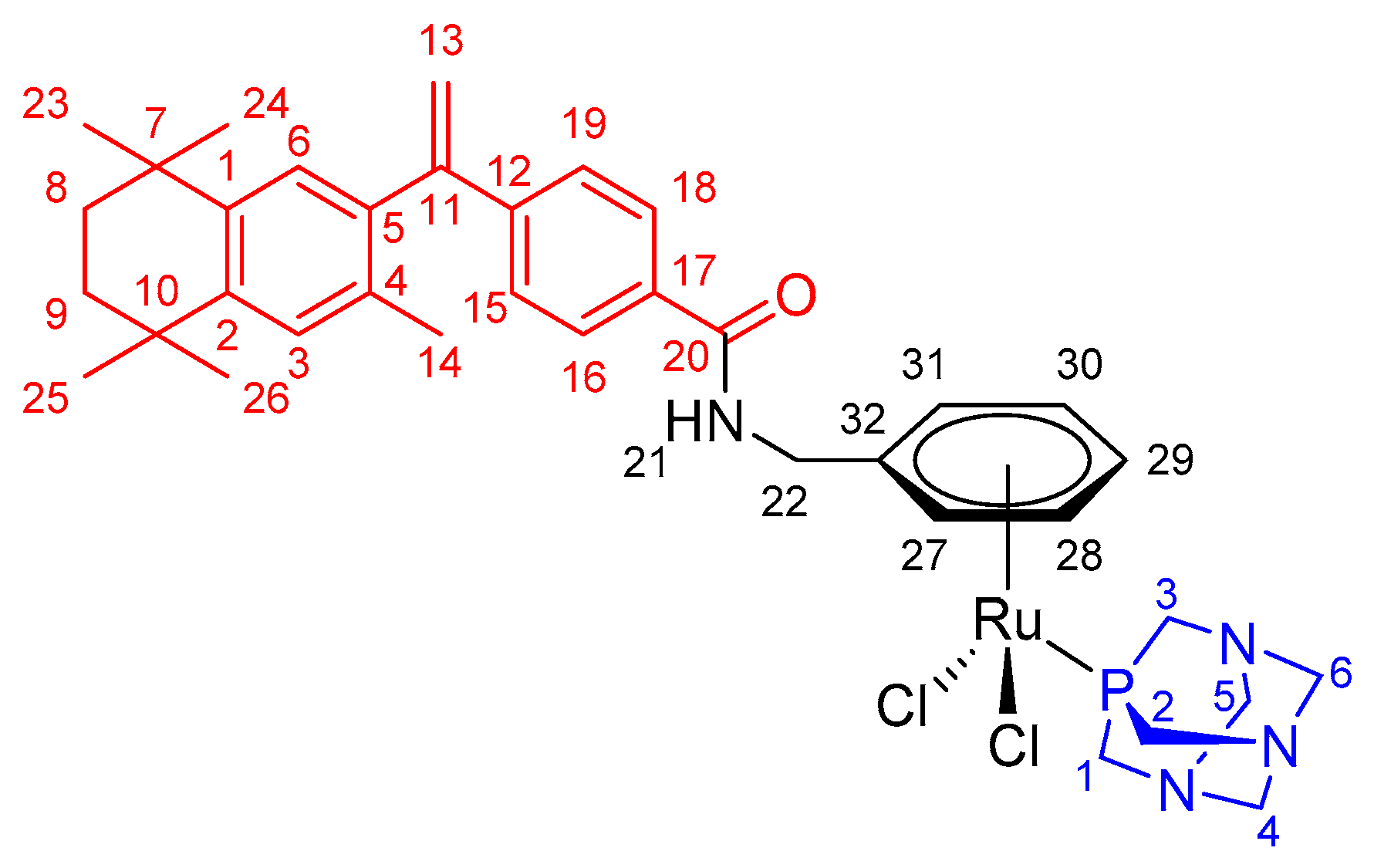
Dichloro[N-[(η6-phenyl)methyl]-4-(1-(3,5,5,8,8-pentamethyl-5,6,7,8tetrahydronaphthalen-2-yl)vinyl)benzamide](1,3,5-triaza-7-phosphatricyclo [3.3.1.13,7]decane-κP7)ruthenium
Abstract
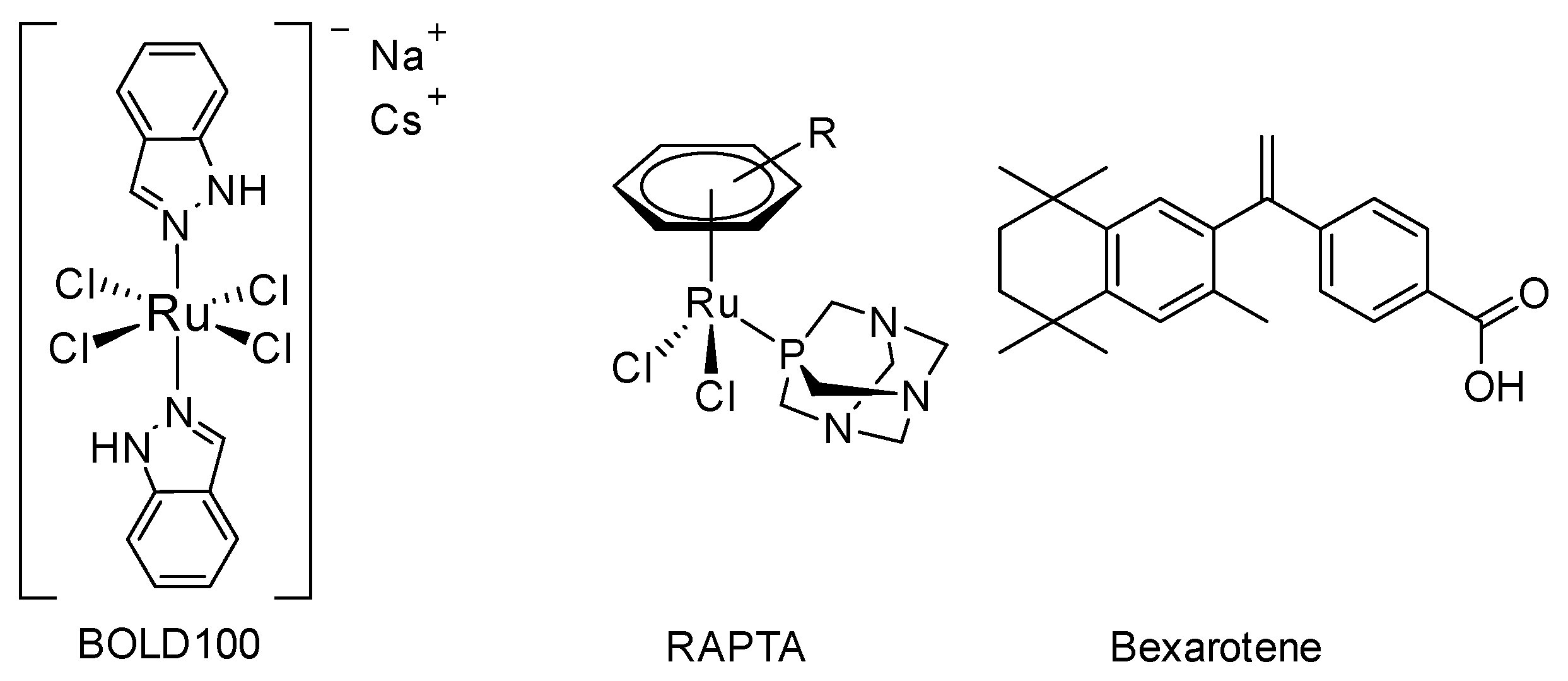
:1. Introduction
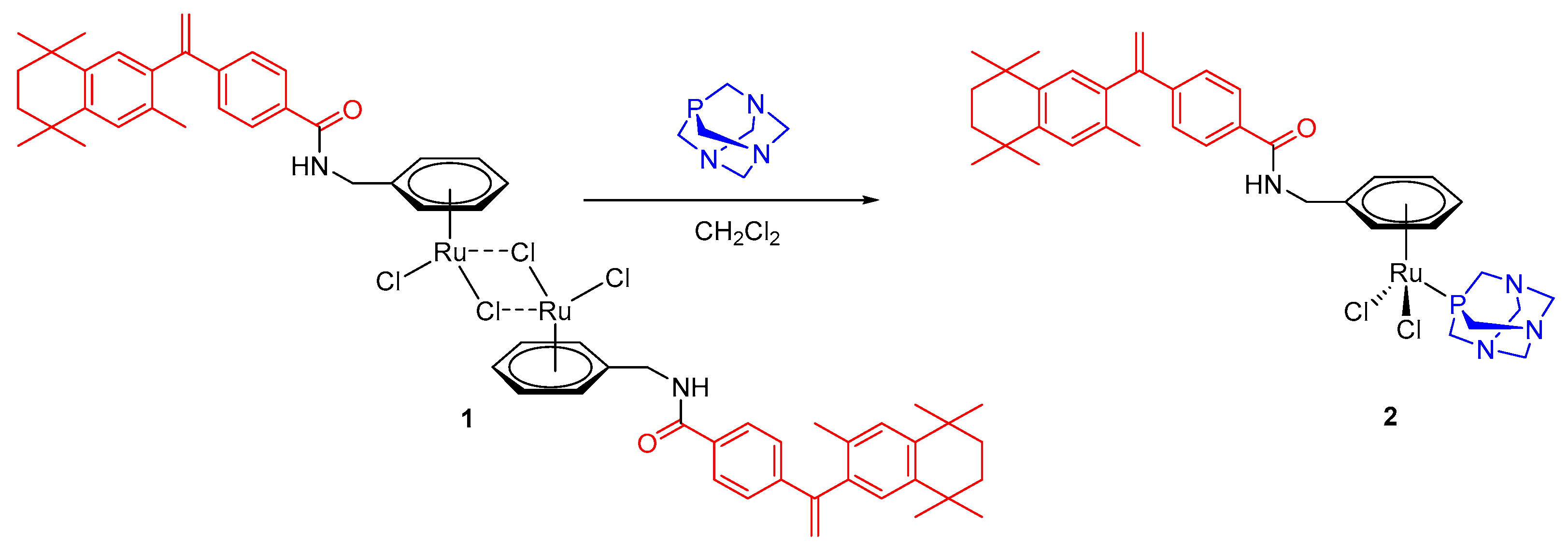
2. Results
3. Materials and Methods
3.1. General
3.2. Cells and In Vitro Antiproliferative Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alessio, E. Thirty Years of the Drug Candidate NAMI-A and the Myths in the Field of Ruthenium Anticancer Compounds: A Personal Perspective. Eur. J. Inorg. Chem. 2017, 2017, 1549–1560. [Google Scholar] [CrossRef]
- Nazarov, A.A.; Hartinger, C.G.; Dyson, P.J. Opening the lid on piano-stool complexes: An account of ruthenium(II)–arene complexes with medicinal applications. J. Organomet. Chem. 2014, 751, 251–260. [Google Scholar] [CrossRef]
- Simpson, P.V.; Desai, N.M.; Casari, I.; Massi, M.; Falasca, M. Metal-based antitumor compounds: Beyond cisplatin. Future Med. Chem. 2019, 11, 119–135. [Google Scholar] [CrossRef] [PubMed]
- FDA Grants Bold Therapeutics’ BOLD-100 an Orphan Drug Designation (ODD) in the Treatment of Gastric Cancer. Available online: https://www.prnewswire.com/news-releases/fda-grants-bold-therapeutics-bold-100-an-orphan-drug-designation-odd-in-the-treatment-of-gastric-cancer-301288416.html (accessed on 11 May 2021).
- Murray, B.S.; Babak, M.V.; Hartinger, C.G.; Dyson, P.J. The development of RAPTA compounds for the treatment of tumors. Coord. Chem. Rev. 2016, 306, 86–114. [Google Scholar] [CrossRef]
- Ang, W.H.; Khalaila, I.; Allardyce, C.S.; Juillerat-Jeanneret, L.; Dyson, P.J. Rational Design of Platinum(IV) Compounds to Overcome Glutathione-S-Transferase Mediated Drug Resistance. J. Am. Chem. Soc. 2005, 127, 1382–1383. [Google Scholar] [CrossRef] [PubMed]
- Kenny, R.G.; Marmion, C.J. Toward Multi-Targeted Platinum and Ruthenium Drugs—A New Paradigm in Cancer Drug Treatment Regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef] [PubMed]
- Nazarov, A.A.; Risse, J.; Ang, W.H.; Schmitt, F.; Zava, O.; Ruggi, A.; Groessl, M.; Scopelitti, R.; Juillerat-Jeanneret, L.; Hartinger, C.G.; et al. Anthracene-Tethered Ruthenium(II) Arene Complexes as Tools To Visualize the Cellular Localization of Putative Organometallic Anticancer Compounds. Inorg. Chem. 2012, 51, 3633–3639. [Google Scholar] [CrossRef] [PubMed]
- Nosova, Y.N.; Karlov, D.S.; Pisarev, S.A.; Shutkov, I.A.; Palyulin, V.A.; Baquié, M.; Milaeva, E.R.; Dyson, P.J.; Nazarov, A.A. New highly cytotoxic organic and organometallic bexarotene derivatives. J. Organomet. Chem. 2017, 839, 91–97. [Google Scholar] [CrossRef]
- Okulova, Y.N.; Zenin, I.V.; Shutkov, I.A.; Kirsanov, K.I.; Kovaleva, O.N.; Lesovaya, E.A.; Fetisov, T.I.; Milaeva, E.R.; Nazarov, A.A. Antiproliferative activity of Pt(IV) complexes with lonidamine and bexarotene ligands attached via succinate-ethylenediamine linker. Inorg. Chim. Acta 2019, 495, 119010. [Google Scholar] [CrossRef]
- Shutkov, I.A.; Antonets, A.A.; Tyurin, V.Y.; Milaeva, E.R.; Nazarov, A.A. Ruthenium(III) Complexes of NAMI-A Type with Ligands Based on Lonidamine and Bexarotene as Antiproliferative Agents. Russ. J. Inorg. Chem. 2021, 66, 502–509. [Google Scholar] [CrossRef]
| Compounds | A549 | HCT116 | MCF7 | SW480 |
|---|---|---|---|---|
| 2 | 31 ± 4 | 32 ± 7 | 27 ± 2 | 23.4 ± 0.6 |
| cisplatin | 8.8 ± 0.9 | 12 ± 2 | 13 ± 1 | 6.2 ± 0.6 |
| Bexarotene | 50 ± 2 | 51 ± 2 | 75 ± 1 | 69 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shutkov, I.A.; Mazur, D.M.; Borisova, N.E.; Milaeva, E.R.; Nazarov, A.A. Dichloro[N-[(η6-phenyl)methyl]-4-(1-(3,5,5,8,8-pentamethyl-5,6,7,8tetrahydronaphthalen-2-yl)vinyl)benzamide](1,3,5-triaza-7-phosphatricyclo [3.3.1.13,7]decane-κP7)ruthenium. Molbank 2022, 2022, M1506. https://doi.org/10.3390/M1506
Shutkov IA, Mazur DM, Borisova NE, Milaeva ER, Nazarov AA. Dichloro[N-[(η6-phenyl)methyl]-4-(1-(3,5,5,8,8-pentamethyl-5,6,7,8tetrahydronaphthalen-2-yl)vinyl)benzamide](1,3,5-triaza-7-phosphatricyclo [3.3.1.13,7]decane-κP7)ruthenium. Molbank. 2022; 2022(4):M1506. https://doi.org/10.3390/M1506
Chicago/Turabian StyleShutkov, Ilya A., Dmitrii M. Mazur, Nataliya E. Borisova, Elena R. Milaeva, and Alexey A. Nazarov. 2022. "Dichloro[N-[(η6-phenyl)methyl]-4-(1-(3,5,5,8,8-pentamethyl-5,6,7,8tetrahydronaphthalen-2-yl)vinyl)benzamide](1,3,5-triaza-7-phosphatricyclo [3.3.1.13,7]decane-κP7)ruthenium" Molbank 2022, no. 4: M1506. https://doi.org/10.3390/M1506
APA StyleShutkov, I. A., Mazur, D. M., Borisova, N. E., Milaeva, E. R., & Nazarov, A. A. (2022). Dichloro[N-[(η6-phenyl)methyl]-4-(1-(3,5,5,8,8-pentamethyl-5,6,7,8tetrahydronaphthalen-2-yl)vinyl)benzamide](1,3,5-triaza-7-phosphatricyclo [3.3.1.13,7]decane-κP7)ruthenium. Molbank, 2022(4), M1506. https://doi.org/10.3390/M1506








