Abstract
The article presents the synthesis and properties of two new Schiff bases of resorcinarene derivatives. The Schiff bases were obtained by the reaction of formylresorcinarene with aromatic (o-aminophenol) and aliphatic (N,N-dimethyldiaminoethane) amines in chloroform. The synthesized Schiff bases exist in equilibrium of several tautomers, as evident from the IR, UV, NMR spectra and cyclic voltammetry data analysis. In DMF, methanol, and acetonitrile, the tautomeric equilibrium is shifted toward the enol-imine tautomers.
Keywords:
schiff base; salicylimine; salen; aminoethylimine; resorcinarene; imine; tautomerism; cyclic voltamperometry 1. Introduction
Schiff bases are a well-known class of organic compounds. They are often used as intermediate and basic components to produce chemicals and new products in industry [1]. Schiff bases exhibit pharmacological properties (antimicrobial, antiviral, antituberculosis, and anticancer activities) and, therefore, they are common in drug development for medicine [2,3,4]. The Schiff bases conjugated to resorcinarene platforms are of greater interest because they combine the properties of both the azomethine groups and the macrocyclic cavity [5,6,7,8]. By organizing four azomethine groups, the resorcinarene platform enhances their ability to form complexes through a synergistic effect [9]. The Schiff bases of resorcinarene derivatives can function as sensors and containers by binding metal ions [10,11,12,13]. Furthermore, a wide spectrum of optical, electromagnetic, catalytic, and pharmacological properties is displayed by the complexes that are created during binding [14,15,16].
In this article we report on two new Schiff bases that are produced by reacting primary aromatic (o-aminophenol) and aliphatic (N,N-dimethyldiaminoethane) amines with the formyl derivative of resorcinarene (fRA, Scheme 1). The structure of the obtained compounds was investigated, and their tautomerism was evaluated by IR, NMR, and UV spectroscopy as well as cyclic voltammetry (CV).
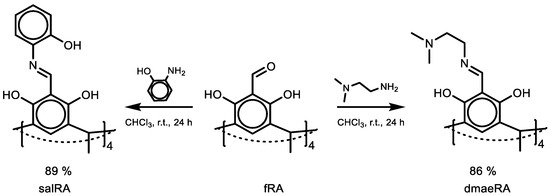
Scheme 1.
Synthesis of salRA and dmaeRA.
2. Results
Formylresorcinarene (fRA) was obtained by the Duff reaction of resorcinarene with urotropine as described in [17]. The reaction was carried out in trifluoroacetic acid (TFA) at 120 °C for 1 h under microwave radiation, then diluted with 1 M hydrochloric acid and stirred vigorously at room temperature for 24 h. The resulting product was purified by repeated washing with water and dried. The yield was 53%.
The condensation of fRA with o-aminophenol and N,N-dimethyldiaminoethane gave two new Schiff bases (salRA and dmaeRA, respectively: Scheme 1). The reaction was carried out in chloroform at room temperature for 24 h. After completion, the products were filtered and washed several times with chloroform. The yield was 89% and 86% for salRA and dmaeRA, respectively.
In the 13C NMR spectrum of salRA, the carbon signal of azomethine appears at 153.98 ppm. In the range of 160–105 ppm, the signals of resorcinarene and phenol rings are fixed (Figure S1 in Supplementary Materials (SM)). In the 1H NMR spectrum of salRA, a peak of the azomethine group occurs at 8.30 ppm, while a signal of a proton intramolecularly bonded between the nitrogen and oxygen atoms NHO appears at 10.50 ppm. A signal of an intramolecularly bound proton between the hydroxyl groups of resorcinarene OHO is observed at 10.17 ppm, while a proton signal of the hydroxyl group of the phenol ring is at 9.05 ppm (Figure S1 in SM). All proton signals are strongly broadened, which is associated with tautomerism. However, the spectra only show one set of signals, which suggests a fast tautomeric exchange [18,19].
A similar picture is observed for dmaeRA (Figure S2 in SM). The azomethine proton is detected in the 1H NMR spectrum at 8.5 ppm, whereas the intramolecularly bound hydrogens of the OH and NH groups are detected at 12.5 and 13.6 ppm. The carbon signal of imine groups appears at 162 ppm in the 13C NMR spectrum, together with the signals of resorcinarene and dimethyldiaminoethane moieties.
SalRA and dmaeRA exist in equilibrium in several tautomeric forms [20,21,22], the structures of which are presented in Scheme 2. The presence of vibration stretching bands of both C=N bonds (1628 cm−1 and 1635 cm−1 for salRA and dmaeRA, respectively) and C=O bonds (1672 cm−1 and 1674 cm−1 for salRA and dmaeRA, respectively) in IR spectra in potassium bromide pellets indicates the formation of both imine- and keto-isomers (Figure 1a,b and Figures S3 and S4 in SM) [23].
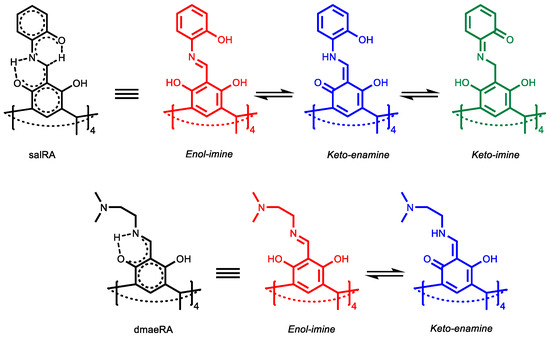
Scheme 2.
Tautomers of salRA and dmaeRA.
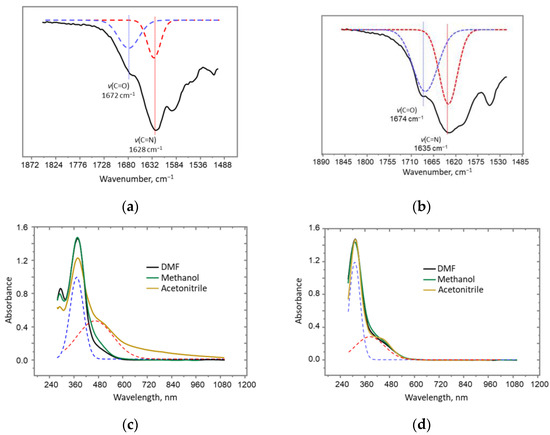
Figure 1.
(a,b) Fragments IR spectra of (a) salRA and (b) dmaeRA; (c,d) UV spectra of (c) salRA and (d) dmaeRA in DMF, methane and acetonitrile (dotted line indicates bands for imine- (blue) and keto-groups (red)), C = 0.04 mM.
The UV spectra of salRA and dmaeRA were studied in the aprotic polar solvents DMF and acetonitrile, as well as in the protic solvent methanol (Figure 1c,d). The UV spectra of all Schiff base solutions show two absorption bands in the 300–500 nm range, which are related to π–π* transitions of the imine- and keto-tautomers [24,25]. For dmaeRA, the absorption band related to the transition of conjugated imine occurs at 330 nm. The band of the keto-enamine tautomer is apparent as a small shoulder at 420 nm, and its optical density is solvent-independent. The conjugation of resorcinolic, phenolic, and azomethynic moieties causes a bathochromic shift of bands in the UV spectra of aromatic salRA (Figure 1c). The band of conjugated imines appears at 375 nm, while the shoulder of ketone moieties occurs at 460 nm. In acetonitrile, an increase in shoulder absorption is observed.
It can be concluded from UV studies that the tautomeric equilibrium in solutions of both Schiff bases is shifted towards the enol-imine tautomers. However, for salRA in acetonitrile, an increase in the proportion of keto tautomers is also detected.
The cyclic voltammetry (CV) of dmaeRA reveals two reduction peaks (C1 and C2) at −1.34 V and −1.83 V, respectively, attributed to the reduction of imine- and keto-groups of enol-imine and keto-enamine tautomers (Figure 2a, Scheme 2). Both peaks were irreversible due to proton transfer from hydroxyl groups to nitrogen and oxygen atoms during the reduction.
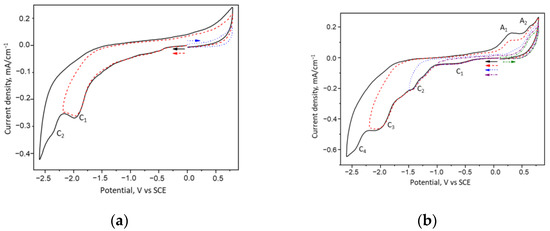
Figure 2.
CV curves of (a) dmaeRA and (b) salRA in DMF/0.1 M Bu4NCl, C = 1 mM, ν = 0.1 V/s.
A more complex picture is observed for salRA (Figure 2a). In this case, four reduction peaks are recorded on the CV. At −2.45 V and −1.99 V (peaks C4 and C3, respectively) irreversible reduction of the imine of the enol-imine tautomer and the quinone of the keto-enamine tautomer occurs (Scheme 2). At potentials of −1.43 V and −1.65 V (peaks C2 and C1), the imine and quinone groups of the keto-imine tautomer are reduced (Figure 2b, Scheme 2). These peaks are reversible, and the reverse scan shows two peaks at 0.30 and 0.62 V from the reoxidation of the reduced o-aminophenol fragments.
Thus, the results of the CV study showed that the obtained Schiff bases exist in equilibrium in several tautomeric forms, caused by the intramolecular movement of protons from hydroxyl groups to azomethines and by the conjugation of resorcinols with azomethine and with aminophenols in the case of salRA. As a result, each tautomer is reduced at a distinct potential, as is evident from the multistage reduction in the CV curves. Such features make compounds promising for the creation of “smart materials”.
3. Materials and Methods
General. NMR spectra were recorded on a Bruker Avance 600 MHz spectrometer. IR spectra were recorded using a Vector-27 FTIR spectrometer (Bruker, Ettlingen, Germany) in the 400–4000 cm−1 range. The samples were prepared as KBr pellets. UV/Vis spectra were recorded with a PerkinElmer Lambda 25 UV/Vis spectrometer. A cuvette with an optical path length of 1 cm was used in all experiments. The elemental analysis was carried out on a CHNS analyzer Vario Macro cube (Elementar Analysensysteme GmbH, Langenselbold, Germany). The samples were weighed on Sartorius Cubis II (Sartorius AG, Gottingen, Germany) microbalance in tin capsules. VarioMacro Software V4.0.11 was used to perform quantitative measurements and evaluate the data received.
Cyclic voltammograms were recorded using a P-30S potentiostat (without IR compensation) at a potential scan rate of 100 mV/s in an argon (99.9999%) atmosphere. The stationary potential or the preliminary keeping potential was set as the initial one. A GC disk electrode (dia. 2.0 mm) sealed into a glass tube was used as the working electrode. Prior to each measurement, the electrode was mechanically polished. A platinum wire was used as the auxiliary electrode. Potentials were measured versus SCE connected to the solution being studied through a bridge containing the supporting electrolyte and having a potential of −0.41 V relative to formal potential E0′ Fc+/0 (internal standard). The temperature was 295 K. Commercial Bu4NCl salt (Sigma-Aldrich, St. Louis, MO, USA) and DMF solvent (Acros Organics, Waltham, MA, USA) were used without additional purification.
Formylresorcinarene (fRA) was synthesized as described in [17].
Synthesis of salRA. A 65.6 mg (0.1 mmol) quantity of formylresorcinarene, and 43.6 mg (0.4 mmol) of o-aminophenol, were placed in a 10 mL vial, and 5 mL of chloroform was added. The mixture was stirred for 24 h at room temperature. The precipitate was separated by centrifugation, washed with chloroform and dried under reduced pressure. Yield: 91 mg (89%). Decomp.p. is 240 °C. The IR spectrum (KBr, ν, cm−1) was: 2966 (C–H), 1672 (C=O), 1628 (C=N), 1459 (C–C). The 1H NMR spectrum (DMSO-d6, ppm) was: 1.76 (d, J = 7.5 Hz, 12H, CH3), 4.64 (qtr, J = 7.4 Hz, 4H, CH), 6. 86, 6.97, 7.11 (m, 12H, Har), 7.59 (d, J = 8.2 Hz, 4H, Har), 7.78 (s, 4H, Har), 8.32 (s, 4H, CH-N), 9.06 (s, 4H, O-H), 10.16 (s, 4H, O–H), 10.50 (s, 4H, N–H–O). The 13C NMR spectrum (DMSO-d6, ppm) was: 18.6, 27.8, 107.8, 116.5, 118.6, 120.6, 124.9, 127.7, 128.6, 133.0, 149.3, 154.0. The anal. calcd. which was for C60H52N4O12 (%) yielded: C, 70.58; H, 5.13; N, 5.49. The amounts that were found (%) were: C, 70.20; H, 5.61; N, 5.32.
Synthesis of dmaeRA. It was synthesized similarly to salRA with the use of 0.5 g (0.76 mmol) of fRA, 0.333 mL (3 mmol) of N,N-dimethylethane-1,2-diamine and 15 mL of chloroform. Yield: 0.61 g (86%). Decomp.p. 220 °C. The IR spectrum (KBr, ν, cm−1) was: 2964 (C–H), 1674 (C=O), 1634 (C=N), 1672 (C=O), 1457 (C–C). The 1H NMR spectrum (DMSO-d6, ppm) was: 1.66 (d, J = 7.5 Hz, 12H, CH3), 2.19 (s, 24H, CH3), 2.50 (m, 8H, CH2), 3.68 (m, 8H, CH2), 4.48 (qtr, J = 7.6 Hz, 4H, CH), 7.57 (s, 4H, Har), 8.57 (s, 4H, CH–N), 12.31 (s, 4H, O–H), 13.68 (s, 4H, NHO). The 13C NMR spectrum (DMSO-d6, ppm) was: 18.4, 27.8, 45.6, 48.2, 58.9, 106.4, 119.0, 132.0, 161.7, 163.0. The anal. calcd. which was for C52H72N8O8 (%) yielded: C, 66.54; H, 7.74; N, 11.96. The amounts that were found (%) were: C, 66.35; H, 7.27; N, 11.87.
4. Conclusions
The two Schiff base derivatives of resorcinarene (salRA and dmaeRA) were produced via condensation of formylresorcinarene with o-aminophenol and N,N-dimethyldiaminoethane, respectively. The resulting Schiff bases have two (in the case of dmaeRA) or three (in the case of salRA) tautomers in equilibrium. The cyclic voltammetry data show that each tautomer is reduced at a defined potential, which is encouraging for the development of materials with particular functionality.
Supplementary Materials
The following supporting information can be downloaded online. Figure S1. 1H and 13C NMR spectra of salRA in DMSO-d6; Figure S2. 1H and 13C NMR spectra of dmaeRA in DMSO-d6; Figure S3. IR spectrum of salRA; Figure S4. IR spectrum of dmaeRA.
Author Contributions
Conceptualization, I.S.A.; methodology, I.S.A. and A.Y.Z.; investigation, O.S.S., R.R.F. and V.V.Y.; resources, I.S.A. and V.V.Y.; data curation, V.V.Y. and A.Y.Z.; writing—original draft preparation, A.Y.Z. and V.V.Y.; writing—review and editing, A.Y.Z. and I.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the government assignment for FRC Kazan Scientific Center of RAS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the Assigned Spectral-Analytical Center of FRC Kazan Scientific Center of RAS for spectral and analytical measurements.
Conflicts of Interest
There are no conflicts to declare.
References
- Dhar, D.N.; Taplo, C.L. Schiff bases and their applications. J. Sci. Ind. Res. 1982, 41, 501–506. [Google Scholar]
- Przybylski, P.; Huczynski, A.; Pyta, K.; Brzezinski, B.; Bartl, F. Biological properties of Schiff bases and azo derivatives of phenols. Curr. Org. Chem. 2009, 13, 124–148. [Google Scholar] [CrossRef]
- Omidi, S.; Kakanejadifard, A. A review on biological activities of Schiff base, hydrazone, and oxime derivatives of curcumin. RSC Adv. 2020, 10, 30186–30202. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.B.; de Fátima, Â. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Hernández-Molina, R.; Mederos, A. Acyclic and macrocyclic Schiff base ligands in comprehensive coordination chemistry ii: From biology to nanotechnology. In Comprehensive Coordination Chemistry II: From Biology to Nanotechnology, 2nd ed.; McCleverty, J.A., Meyer, T.J., Eds.; Pergamon: London, UK, 2003; Volume 1, pp. 411–446. [Google Scholar]
- Gajjar, J.A.; Vekariya, R.H.; Sharma, V.S.; Kher, S.N.; Rajani, D.P.; Parekh, H.M. Mesomorphic properties, microwave-assisted synthesis, and antimicrobial evaluation of novel Schiff base functionalized resorcin [4] arene derivatives. Mol. Cryst. Liq. Cryst. 2021, 715, 37–55. [Google Scholar] [CrossRef]
- Jayswal, K.P.; Patel, J.R.; Patel, V.B.; Patel, M.H. A new approach towards synthesis of some novel “upper rim” functionalized calix[4]resorcinarene Schiff-bases. Acta Chim. Slov. 2008, 55, 302–307. [Google Scholar]
- Ge, Y.; Yan, C. Rapid synthesis of calix[4]resorcinarene-based dendrimers containing salicylideneimine terminal groups. J. Chem. Res. 2004, 2004, 279–281. [Google Scholar] [CrossRef]
- Vicens, J.; Harrowfield, J.; Baklouti, L. Calixarenes in the Nanoworld; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Upadhyay, J.B.; Parekh, H.M. Resorcin[4]arene Schiff base derivatives: Synthesis, characterization, and extraction studies. J. Chem. Res. 2020, 44, 660–666. [Google Scholar] [CrossRef]
- Neri, P.; Sessler, J.L.; Wang, M.-X. Calixarenes and Beyond; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar]
- Baldini, L.; Casnati, A.; Sansone, F.; Ungaro, R. Calixarene-based multivalent ligands. Chem. Soc. Rev. 2007, 36, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Quang, D.T. Calixarene-derived fluorescent probes. Chem. Rev. 2007, 107, 3780–3799. [Google Scholar] [CrossRef]
- Uddin, M.N. Biomedical applications of Schiff base metal complexes. J. Coord. Chem. 2020, 73, 3109–3149. [Google Scholar] [CrossRef]
- Catalano, A.; Sinicropi, M.S.; Iacopetta, D.; Ceramella, J.; Mariconda, A.; Rosano, C.; Scali, E.; Saturnino, C.; Longo, P. A review on the advancements in the field of metal complexes with schiff bases as antiproliferative agents. Appl. Sci. 2021, 11, 6027. [Google Scholar] [CrossRef]
- Creaven, B.S.; Donlon, D.F.; McGinley, J. Coordination chemistry of calix[4]arene derivatives with lower rim functionalisation and their applications. Coord. Chem. Rev. 2009, 253, 893–962. [Google Scholar] [CrossRef]
- Eichstaedt, K.; Szpotkowski, K.; Grajda, M.; Gilski, M.; Wosicki, S.; Jaskjlski, M.; Szumna, A. Self-assembly and ordering of peptide-based cavitands in water and DMSO: The power of hydrophobic effects combined with neutral hydrogen bonds. Chem. Eur. J. 2019, 25, 3091–3097. [Google Scholar] [CrossRef] [PubMed]
- Pizzala, H.; Carles, M.; Stone, W.E.E.; Thevand, A. Tautomerism in Schiff bases derived from 3-hydroxysalicylaldehyde. Combined X-ray diffraction, solution and solid state NMR study. J. Chem. Soc. Perkin Trans. 2000, 2, 935–939. [Google Scholar] [CrossRef]
- Dobosz, R.; Mućko, J.; Gawinecki, R. Using Chou’s 5-step rule to evaluate the stability of tautomers: Susceptibility of 2-[(phenylimino)-methyl]-cyclohexane-1,3-diones to tautomerization based on the calculated Gibbs free energies. Energies 2020, 13, 183. [Google Scholar] [CrossRef]
- Martínez, R.F.; Ávalos, M.; Babiano, R.; Cintas, P.; Jiménez, J.L.; Light, M.E.; Palacios, J.C. Tautomerism in Schiff bases. The cases of 2-hydroxy-1-naphthaldehyde and 1-hydroxy-2-naphthaldehyde investigated in solution and the solid state. Org. Biomol. Chem. 2011, 9, 8268–8275. [Google Scholar] [CrossRef]
- Grajda, M.; Wierzbicki, M.; Cmoch, P.; Szumna, A. Inherently Chiral Iminoresorcinarenes through Regioselective Unidirectional Tautomerization. J. Org. Chem. 2013, 78, 11597–11601. [Google Scholar] [CrossRef]
- Jędrzejewska, H.; Kwit, M.; Szumna, A. Switching of inherent chirality driven by self-assembly. Chem. Commun. 2015, 51, 13799–13801. [Google Scholar] [CrossRef] [PubMed]
- Salman, S.R.; Saleh, N.A.I. Infra-red study of tautomerism in some Schiff bases. Spectrosc. Lett. 1997, 30, 1289–1300. [Google Scholar] [CrossRef]
- Atzin-Macedo, C.M.; Pastor-Ramírez, C.; González-Peláez, R.; Pérez-Flores, F.J.; Hernández-Anzaldo, S.; Vazquez-Lima, H.; Reyes-Ortega, Y. Tautomeric study of Schiff bases derived from o-dihydroxybenzaldehyde by UV-Vis, IR, 1H NMR, 13C NMR spectroscopy and computational modeling. ChemistrySelect 2020, 5, 11120–11126. [Google Scholar] [CrossRef]
- Minkin, V.I.; Tsukanov, A.V.; Dubonosov, A.D.; Bren, V.A. Tautomeric Schiff bases: Iono-, solvato-, thermo- and photochromism. J. Mol. Struct. 2011, 998, 179–191. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).